| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Nucleophilic Substitution
Answers
| Qu 1 | The first step has to be identifying the
reaction involved. This is a nucleophilic substitution of the methyl iodide.
This conclusion could by reacted by either reflecting on the chemistry of
alkyl halides or how bad -NH2 would be as a leaving group or
that the N atom has lone pairs that make it a potential nucleophile.
In both cases the reaction leads to N-methylamines via an SN2 reaction. Methyl systems do not undergo SN1 reactions (the carbocation is too high energy). Knowing what the reaction is, we can then determine that the amine species are the nucleophiles in those reactions. For aniline, resonance of the N lone pair with the adjacent π system decreases the availability if the lone pair and so makes aniline a poorer nucleophile thus resulting in the lower reactivity. |
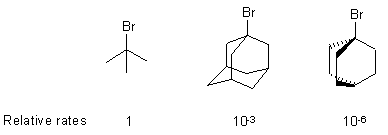
| Qu 2 | The relative reactivity of the following
tertiary alkyl bromides towards hydrolysis in aqueous ethanol solution (favours
SN1) is due to the requirement for planarity in the carbocation.
The 2 ring systems on the right cannot become planar due to steric effects
and the cations are not as stable. Hence, they react more slowly. They are
prohibited from SN2 reaction by the ring system preventing
the "backside" attack.
|
| Qu 3 |
|
| Relatvie nucleophilicity depends on electron availablitity. Factors to consider are the charge and the identify of the atom carrying the charge. Remember an organolithium is essentially a carbanion as is very reactive. S is more nucleophilic than O because of its polarizability. The electronegativity of F causes inductive electron withdrawal, lowering the electron density on O, but not to the same degree as the resonance stabilisation in the carboxylate. The neutral O of the ether is last. |
|
| Qu 4 | We need to use the idea that a good leaving group is the conjugate base of a strong acid, since it infers that the displaced group will be stable: |
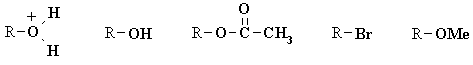
How would we rationalise the pKa's of these or rank their acidity ? HBr is a very strong acid since the bromine is very capable of accommodating negative charge. Hydronium is also a strong acid with a neutral conjugate base. Carboxylic acids are weak acids, but the resonance stabilisation of the carboxylate makes them a strong acid than an alcohol. Alcohols and water have very similar acidity. R-Br (HBr = -9) > R-O+H2 (H3O+ = -1.7) > RO2CCH3 (CH3CO2H = 4.8) > ROMe (MeOH = 15.5) = ROH (H2O = 15.7) |
|
| Qu 5 |
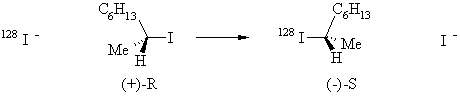
This is just a simple nucleophilic substitution reaction, with a couple of subtleties. First since the Nu = LG, the starting material and the products have equal chemical reactivity towards the iodide, thus, regardless of it being SN1 or SN2 the reaction mixture will always eventually become racemic (50% R, 50%S). since the (S)-product is the enantiomer of the (R)-starting material. The rate of iodine incorporation tells us how fast the iodide becomes part of the product, the rate of racemisation tells us how rapidly the 50:50 mixture is formed......these two things are not quite the same. If the reaction is SN1, then the planar carbocation intermediate will mean the both (R)- and (S)-products will be formed in equal amounts. This means that when two molecules of the (R)-starting material react, one will give (R)-product and the other (statistically) will give one of (S)-product. The rotations of these will cancel each other out. So this would mean that the rate of iodide incorporation would equal the rate of racemisation. If the reaction is SN2, then inversion occurs, so only the (S)-product will be formed. This means that for every molecule of (R)-starting material that reacts, one molecule of (S)-product is produced. This (S)-enantiomer will also cancel out the rotation of another (R)-starting material. This means that the rate of iodide incorporation would equal the half rate of racemisation since each iodide converts an (R) to an (S) that cancels out the rotation of a second (R) molecule. Therefore the reaction must be occurring via the SN2 pathway. |
| Qu 6 | |
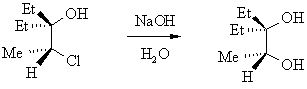 (substitution with retention of stereochemistry) (substitution with retention of stereochemistry)
Even though we have not yet seen all ot these reactions, given that you have the structure of the reactants and the products, you should be able to draw a mechanism to show the formation of the product based on what you know from earlier chapters. If this reaction were SN1 then we would have loss of stereochemistry due to the carbocation intermediate. If the reaction were simple SN2, then there should have been inversion....one way to get retention is to have two inversions.... So if the HO- is not a nucleophile, then it reacts as a base, generating the alkoxide, RO-, another potential nucleophile. This reacts SN2 fashion intramolecularly to displace the Cl- with inversion, giving an epoxide.
|
|
| Qu 7 | The question is based on the radical halogenation of a hydrocarbon H followed by an analysis of the SN1 or SN2 reactivity of the chloroalkanes. The possible isomeric structures for H are: |

The fact that 4 monochlorinated products were obtained
tells us that the original hydrocarbon must have contained 4 types of
aliphatic H.
The AgNO3 / EtOH / H2O reaction is SN1 and tells us the relative stability of the carbocations, this helps decide which structure is which. C will be fastest since it gives a tertiary carbocation, A and B give the same secondary carbocation and D will the slowest as it is only primary. The NaI / acetone reaction is SN2 and tells us about the steric hindrance at the Cl center. D is fastest as it is primary, C is the slowest as it is tertiary. A is faster than B because the methyl group in B will hinder the approach of the nucleophile at 180o to the leaving group during the inversion. A = cis-1-chloro-2-methylcyclopropane, or (1S,2R)-1-chloro-2-methylcyclopropane
(as drawn) If bromination were used, the major product would
be 1-bromo-1-methylcyclopropane because radical bromination reactions
are much more selective and can be predicted based on the most stable
radical which would the tertiary radical. |
|
| Qu 8 | |
| The mechanism is a substitution reaction of an alcohol
with HCl to give a re-arranged chloroalkane (that is the new substituent
is not attached to the same carbon skeleton as the starting material). The
fact that the carbon skeleton rearranges indicates that a carbocation was
formed, and therefore, that the process is SN1 rather
than SN2. The key parts are protonating the -OH to make a good leaving group (-OH will not act as a leaving group to give hydroxide), loss of the leaving group (H2O) to give the secondary carbocation, rapid rearrangement to the more stable tertiary carbocation and attack by the chloride nucleophile. An SN2 reaction would have given the un-rearranged product, 2-chloro-3,3-dimethylbutane.
|
| © Dr. Ian Hunt, Department of Chemistry |