| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Summary
What does the term "nucleophilic substitution" imply ?
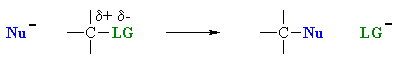
Nucleophilic substitution reactions are an important class of reactions that allow the interconversion of functional groups.
Of particular importance are the reactions of alkyl halides (R-X) and alcohols (R-OH)
For alcohols, the range of substitution reactions possible can be increased by utilising the tosylates (R-OTs), an alternative method of converting the -OH to a better leaving group.
Nucleophilic Substitution
| © Dr. Ian Hunt, Department of Chemistry |