| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Stability:
The general stability order of simple alkyl carbocations is: (most stable) 3o
> 2o > 1o > methyl (least stable)
![[carbocation stability order]](c+stab.gif)
This is because alkyl groups are weakly electron donating
due to hyperconjugation and inductive effects.
Resonance effects can further stabilise carbocations when present (therefore allyl or benzyl carbocations are more stable than simple primary carbocations).
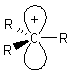
|
Alkyl carbocations are sp2 hybridised, planar
systems at the cationic C centre. The p-orbital that is not utilised in the hybrids is empty and is often shown bearing the positive charge since it represents the orbital available to accept electrons. |
|
 |
As they have an incomplete octet, carbocations are
excellent electrophiles and react readily with nucleophiles. Alternatively,
loss of H+ can generate a p bond.
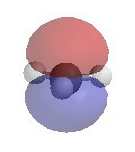
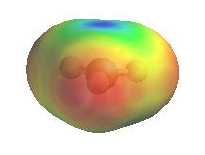
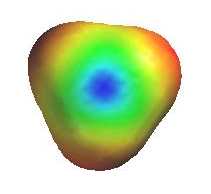
The electrostatic potential diagrams clearly show
the cationic center in blue, this is where the nucleophile will attack.
|
 |
Rearrangements:
Carbocations are prone to rearrangement via 1,2-hyride or 1,2-alkyl shifts provided
at each step, a more stable carbocation is generated. For example:
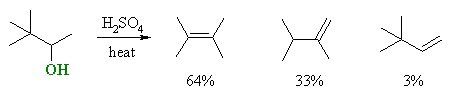
|
|
| Notice in this example that the product we might initially "predict" based on a simple application of Zaitsev's rule, is only formed
in 3% yield, and instead, products with a different skeleton (i.e. a different hydrocarbon backbone) dominate.
The reaction proceeds via protonation to give the better leaving group which departs to give the 2o carbocation shown. A methyl group rapidly migrates taking its bonding electrons along, giving a new skeleton and a more stable 3o carbocation which can then lose H+ to give the more stable alkene as the major product. |
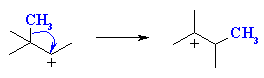 2o carbocation to 3o carbocation |
This is an example of a 1,2-alkyl shift. The
numbers indicate that the alkyl group moves to an adjacent position.
Similar migrations of H atoms, 1,2-hydride shifts are also known.
A good rule of thumb for carbocations....."If they can rearrange, they will" (that means if a more stable carbocation can be formed, then expect them to rearrange.
Rearrangments of simple alkyl carbocations are of the 1,2-type (this means that only a group on an adjacent position migrates).
As a potential sign of rearrangement, look for carbocations adjacent to branch points (see the example above). Carbocations in the middle of unbranched chains will not usually rearrange.
If a product seems to have a different hydrocarbon skeleton, that is an indicator to check for a carbocation rearrangement via alkyl shifts (see above).
If a product seems to have a new functional group in a new location, that is an indicator to check for a carbocation rearrangement via a hydride shift.
Reactions involving carbocations:
1. Substitutions via SN1 (see also Chapter
4)
2. Eliminations via E1 (Chapter 5)
3. Additions to alkenes (e.g. HX, H3O+)
(Chapter 6)
4. Additions to alkynes (e.g. HX, H3O+)
(Chapter 9)
5. Friedel-Crafts alkylations (Chapter 12)
| © Dr. Ian Hunt, Department of Chemistry |