|
|
|
|
Friedel-Crafts Alkylation of Benzene
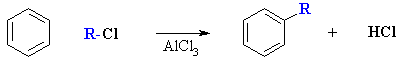
Reaction type: Electrophilic Aromatic Substitution
Summary
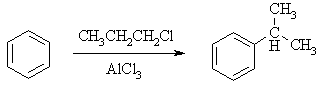
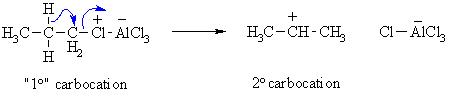
Summary of Limitations of Friedel-Crafts alkylations:
|
|
|
|
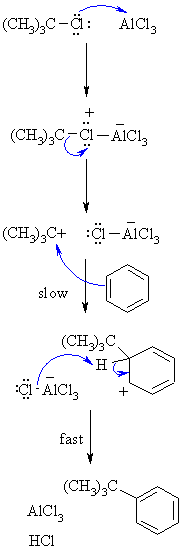
Step 1:
Step 2:
Step 4: |
|
| © Dr. Ian Hunt, Department of Chemistry |