|
|
|
|
Halogenation of Benzene
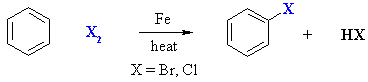
Summary
|
|
|
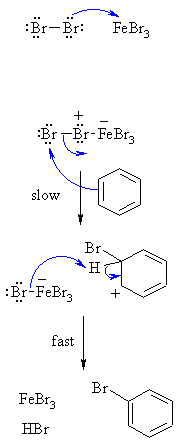
| Step 1: The bromine reacts with the Lewis acid to form a complex that makes the bromine more electrophilic. |
|
| Step 2: The p electrons of the aromatic C=C act as a nucleophile, attacking the electrophilic Br, and displacing iron tetrabromide. This step destroys the aromaticity giving the cyclohexadienyl cation intermediate. |
|
| Step 3: Removal of the proton from the sp3 C bearing the bromo- group reforms the C=C and the aromatic system, generating HBr and regenerating the active catalyst. |
|
| © Dr. Ian Hunt, Department of Chemistry |