| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
SN1 indicates a substitution, nucleophilic,
unimolecular reaction, described by the expression rate =
k [R-LG].
This implies that the rate determining step of the mechanism depends on the
decomposition of a single molecular species.
This pathway is a multi-step process with the following
characteristics:
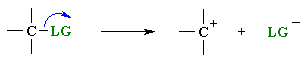 |
step 1: slow loss of the leaving group, LG, to generate a carbocation intermediate, then | |
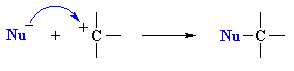 |
step 2 : rapid attack of a nucleophile on the electrophilic carbocation to form a new s bond |
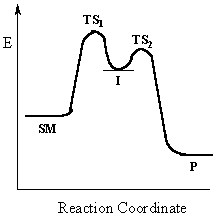 |
Multi-step reactions have intermediates and a several
transition states (TS).
In an SN1 there is loss of the leaving group generates an intermediate carbocation which is then undergoes a rapid reaction with the nucleophile..
|
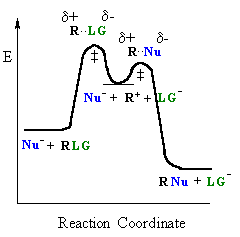 |
| General case | SN1 reaction |
Let's look at how the various components of the reaction influence the reaction pathway:
R-
Reactivity order : (CH3)3C- >
(CH3)2CH- > CH3CH2-
> CH3-
In an SN1 reaction, the rate determining step
is the loss of the leaving group to form the intermediate carbocation. The more
stable the carbocation is, the easier it is to form, and the faster the SN1
reaction will be. Some students fall into the trap of thinking that the
system with the less stable carbocation will react fastest, but they are forgetting
that it is the generation of the carbocation that is rate determining.
Since a carbocation intermediate is formed, there is the possibility of rearrangements
(e.g. 1,2-hydride or 1,2-alkyl shifts) to generate a more stable carbocation.
This is usually indicated by a change in the position of the substituent or
a change in the carbon skeleton of the product when compared to the starting
material.
The following JSMOL images show a series of alkyl bromides
and their relative rates of reaction in an SN1 hydrolysis.
Try to correlate the structure of the alkyl bromide with the type of carbocation
that will be formed.
If you need help, click the button to show you where the carbocation would be
formed - it will highlight the electrophilic center.
|
|
|
|
|
|
| Relative rate of hydrolysis | 1 |
2 |
43 |
100,000,000 |
-LG
The only event in the rate determining step of the SN1 is breaking
the C-LG bond. Therefore, there is
a very strong dependence on the nature of the leaving group, the better the
leaving, the faster the SN1 reaction will be.
Nu
Since the nucleophile is not involved in the rate determining step, the nature
of the nucleophile is unimportant in an SN1 reaction. However, the
more reactive the nucleophile, the more likely an SN2 reaction becomes.
Stereochemistry
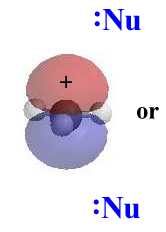 |
In an SN1, the nucleophile attacks the
planar carbocation. Since there is an equally probability of attack on
each face there will be a loss of stereochemistry at the reactive
center as both products will be observed.
|
Solvent
Polar solvents which can stabilise carbocations which can favour the SN1
reaction (e.g. H2O, ROH)
Summary
This pathway is most common for systems with good leaving groups, stable carbocations
and weaker nucleophiles. A typical example is the reaction of HBr with a tertiary
alcohol.
| SN1 MECHANISM FOR REACTION OF ALCOHOLS WITH HBr | |
| Step 1: Step 2: Step 3:
|
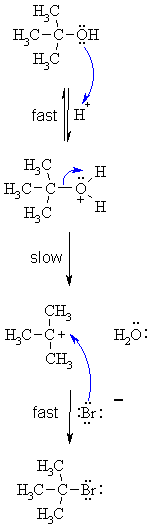 |
| SN1 MECHANISM FOR REACTION OF ALKYL HALIDES WITH H2O | |
| Step 1: Step 2: Step 3: Note that this is the reverse of the reaction of an alcohol with HBr. In principle, the nucleophile here, H2O,
could be replaced with any nucleophile, in which case the final deprotonation
may not always be necessary. |
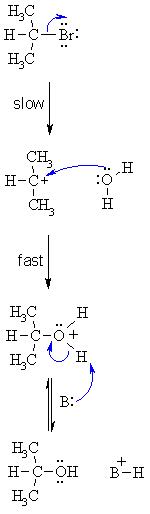 |
| © Dr. Ian Hunt, Department of Chemistry |