| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Substitution versus Elimination
Substitution and elimination reactions often compete with each other because it's a question of nucleophilic or basic properties.
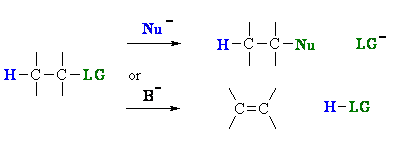
Substitution and elimination reactions are strongly influenced by many experimental factors.
1. Increasing the temperature tends to increase elimination due to disorder / entropy effects (recall DG - DH -TDS)
2. Increasing steric effects (look at both "R" and the "Nu/B") tends to increase elimination.
3. The basicity / nucleophilicity of the attacking species i.e. switching from ROH to RO- will increase the amount of elimination
Some of more important mechansitic factors are outlined in the following
table.
In the table, the significance of the effect is stated first, and then the "system" that will favour
the reaction is stated.
This should help you deal with the questions....
1. When does an anion function as a Nu and when does it function as a B ?, and
therefore,
2. When to I get substitution and when do I get elimination ?
| Reaction | Substrate | Nu or Base | Leaving Group | Solvent | Examples |
| SN1 | Strong 3o or resonance stabilised |
Weak Good Nu and weak base |
Strong Good LG |
Very Strong Polar solvents |
alkyl halide / AgNO3 / aq. EtOH alcohol / HX (note X is a good Nu) |
| SN2 | Strong Methyl or 1o |
Strong Good Nu and weak base |
Strong Good LG |
Strong Polar aprotic solvents |
alkyl halide / NaI / acetone alcohol / SOCl2 or PCl3 |
| E1 | Strong 3o or resonance stabilised |
Weak Weak base |
Strong Good LG |
Very Strong Polar solvents |
alcohol / H2SO4 / heat tertiary alkyl halide / weak base (ROH or H2O) |
| E2 | Strong 3o |
Strong Poor Nu and strong base |
Strong Good LG |
Strong Polar aprotic solvents |
alkyl halide / KOH / heat, primary alcohols / alcohol / H2SO4 / heat |
| © Dr. Ian Hunt, Department of Chemistry, University of Calgary |