| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
π Molecular Orbitals
Qualitative Molecular Orbital theory is a fascinating aspect of organic chemistry that can provide a remarkable insight into the workings of organic reactions based on how orbitals interact to control the outcome of reactions. The ideas were developed by Fukui, Hoffmann and Woodward, leading to a Nobel Prize for Fukui and Hoffmann in 1981.
An introduction to the basic components is presented here. A more thorough analysis probably requires another semester !
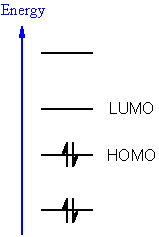 |
LUMO lowest
unoccupied molecular orbital |
|
| HOMO highest
occupied molecular orbital |
|
| © Dr. Ian Hunt, Department of Chemistry |