| Chapter 11 : Arenes and Aromaticity |
| Chapter 11 : Arenes and Aromaticity |
What about Other Neutral Hydrocarbons ?
The following table contains two hydrocarbons that have been notably absent so far in our discussions even though both are cyclic and have alternating C=C and C-C, but they contain either one less or one more C=C than benzene. These compounds are 1,3-cyclobutadiene and 1,3,5,7-cyclooctatetraene.
Experimental evidence such as reactivity, spectroscopic
data and thermodynamic measurements suggest that 1,3,5,7-cyclooctatetraene is
not aromatic but is more like a conjugated polyene.
The experimental evidence tells us:
Interestingly, an analysis of the heat of hydrogenation of cyclooctatetraene (410 kJ/mol or 98 kcal/mol) compared to cyclooctene (97 kJ/mol or 23 kcal/mol) indicates that the tetraene is actually slightly destabilised by about 22kJ/mol (5 kcal/mol). This certainly confirms that cyclooctetraene isn't aromatic.
In terms of the aromaticity criteria described earlier, cyclooctatetraene is not aromatic since it fails to satisfy the 4n + 2 π electron Huckel rule (i.e. it doesn't have an odd number of π electron pairs). It is actually an example of a 4n π electron system (i.e. an even number of π electron pairs). So, if cyclooctatetrene were planar it would be anti-aromatic, a destabilising situation. Molecules tend to try to adopt more stable shapes: think of cyclohexane being in the chair conformation rather than planar or the anti conformation of alkyl chains. Cyclooctatetraene prefers to adopt a more stable non-planar conformation (which also helps alleviate some angle strain : an octagon has internal angles of 135 degrees while sp2 C require 120 degrees).
Since cyclooctatetraene violates one of the first three aromaticity criteria (it's not planar), it is best described as non-aromatic.
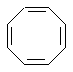 Cyclooctatetraene |
|
Resonance energy CC bonds 1.35 and 1.5 A H NMR δH = 5.7 ppm |
The fact that 1,3-cyclobutadiene is very difficult to prepare due to its very high reactivity tells us that unlike benzene, 1,3-cyclobutadiene is not a particularly stable compound. The evidence suggests cyclobutadiene has CC bonds of different lengths (i.e. alternating C=C and C-C). In terms of the aromaticity criteria described earlier , 1,3-cyclobutadiene is not aromatic since it fails to satisfy the 4n + 2 π electron Huckel rule (i.e. it doesn't have an odd number of π electron pairs). Infact, it is an example of a 4n π electron system (i.e. an even number of π electron pairs). However, since cyclobutadiene violates the final aromaticity criteria, it is described as anti-aromatic. This term is used to reflect its lack of stability and high reactivity.
|
Cyclobutadiene |
| © Dr. Ian Hunt, Department of Chemistry |