| Chapter 14: Organometallic Compounds |
| Chapter 14: Organometallic Compounds |
| Qu 1: | Remember, organometallic compounds contain carbon-metal bonds | ||||||||
| (a) CH3CH2ONa
: No, since it is an oxygen-metal bond (b) CH3CH2Li : Yes there is a carbon-metal bond (c) CH3CH2BH2 : No, boron is a non-metal (d) (CH3CH2)2Zn : Yes there are 2 carbon-metal bonds (e) CH3CH2MgBr : Yes there is a carbon-metal bond (f) CH3C≡CNa : Yes there is a carbon-metal bond |
|||||||||
| Qu 2: | (c) Metals are electropositive and carbon is electronegative with respect to a metal. | ||||||||
| Qu 3: | CH3CH2- > CH2=CH- > CH3C≡C- > CH3CH2O- > CH3CH2OH (check pKa's) | ||||||||
| A good way to think
of basicity is the ability of the basic atom to donate it's pair of electrons
(Lewis definition). Factors such as electronegativity and hybridisation are important. Electronegative atoms are poor electron donors since they hold onto their electrons. sp orbitals are more electronegative then sp2 due to the higher "s" character. Likewise for sp2 over sp3. |
|||||||||
| Qu 4: | The Grignard reagent CH3CH2MgBr gives the following products: | ||||||||
|
|||||||||
| Qu 5: | Cuprates are prepared by adding Cu (I) halides to organolithium reagents, so we must first prepare the organolithium reagent. | ||||||||
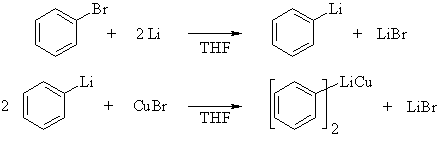
|
|||||||||
| Qu 6: | The cuprate (c) is the best choice. Organolithiums and Grignard reagents are too basic for coupling reactions for alkane synthesis. Organozinc reagents are not reactive enough. | ||||||||
| Qu 7: | |||||||||
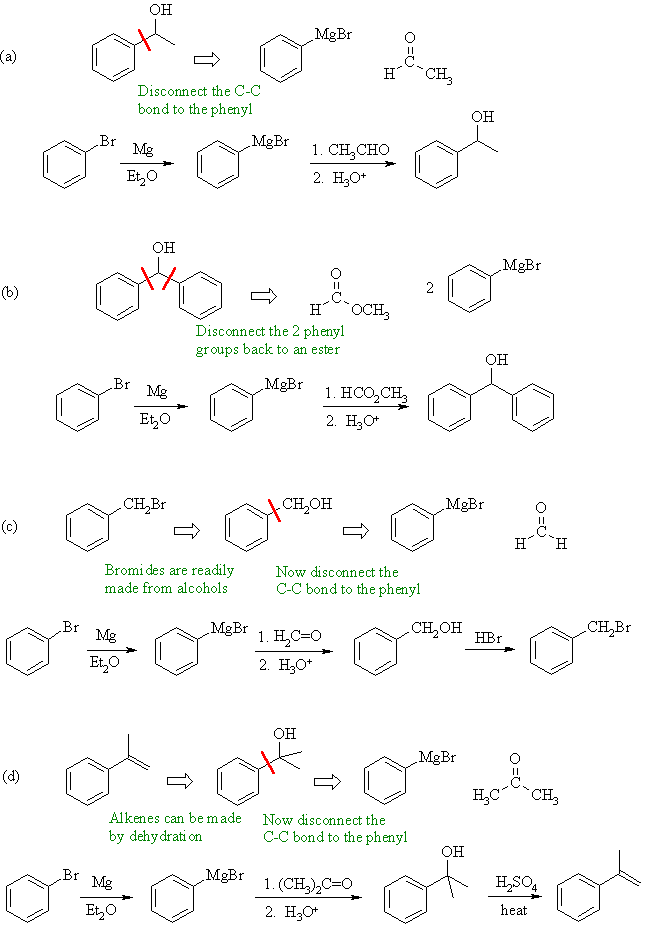
|
| © Dr. Ian Hunt, Department of Chemistry |