|
|
|
|
The Wittig Reaction
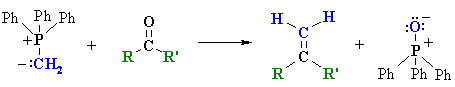 |
| ylide aldehyde or ketone alkene |
Reaction type: Nucleophilic Addition then Elimination
Summary
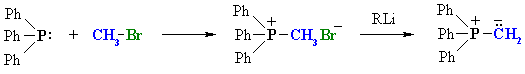
|
| An SN2 reaction between triphenyl phosphine and an alkyl halide followed by treatment with a strong base such as an organolithium reagent. |
Related Reactions
THE WITTIG REACTION |
|
| Step 1: The nucleophilic C of the ylid Wittig reagent adds to the electrophilic C in the polar carbonyl group, electrons from the C=O π bond are used to form a σ bond to the +ve P atom. This creates a cyclic intermeiate called an oxaphosphetane. |
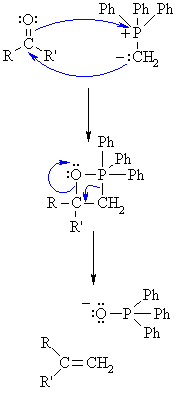 |
Step 2:
|
|
| © Dr. Ian Hunt, Department of Chemistry |