| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
Formation of Hydrates
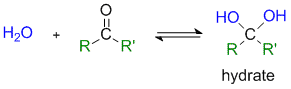 |
Summary
MECHANISM
FOR THE ACID CATALYSED FORMATION OF HYDRATES |
|
| Step 1: An acid/base reaction. Since there is only a weak nucleophile we need to activate the carbonyl by protonating on O. |
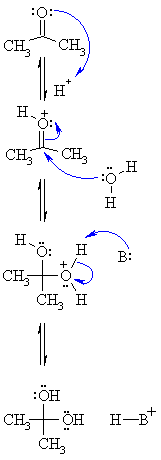 |
| Step 2: The nucleophilic O in the water attacks the electrophilic C in the C=O, breaking the π bond and giving the electrons to the positive O |
|
| Step 3: An acid/base reaction. Deprotonation of the oxonium ion neutralises the charge giving the hydrate. |
|
| © Dr. Ian Hunt, Department of Chemistry |