| Chapter 20: Carboxylic Acid Derivatives. NucleophilicAcyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. NucleophilicAcyl Substitution |
Hydrolysis of Esters
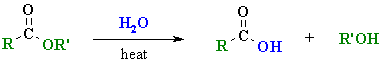
Reaction type: Nucleophilic Acyl Substitution
Summary
|
|
|
Step 1: The hydroxide nucleophiles attacks at the electrophilic C ofthe ester C=O, breaking the π bond and creating the tetrahedral intermediate. |
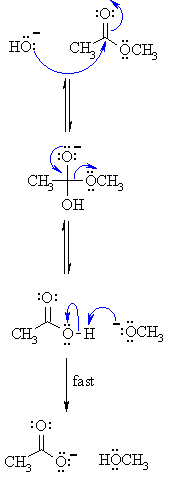
|
| Step 2: The intermediate collapses, reforming the C=O results in the loss of the leaving group the alkoxide, RO-, leading to the carboxylic acid. |
|
| Step 3: An acid / base reaction. A very rapid equilibrium where the alkoxide,RO- functions as a base deprotonating the carboxylic acid, RCO2H, (an acidic work up would allow the carboxylic acid to be obtained from the reaction). |
|
Reaction under ACIDIC conditions:
|
|
|
| Step 1: An acid/base reaction. Since we only have a weak nucleophile and a poor electrophile we need to activate the ester. Protonation of the ester carbonyl makes it more electrophilic. |
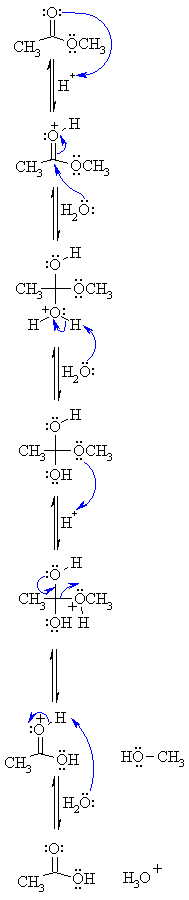
|
| Step 2: The water O functions as the nucleophile attacking the electrophilic C in the C=O, with the electrons moving towards the oxonium ion, creating the tetrahedral intermediate. |
|
| Step 3: An acid/base reaction. Deprotonate the oxygen that came from the water molecule to neutralise the charge. |
|
| Step 4: An acid/base reaction. Need to make the -OCH3 leave, but need to convert it into a good leaving group first by protonation. |
|
| Step 5: Use the electrons of an adjacent oxygen to help "push out" the leaving group, a neutral methanol molecule. |
|
| Step 6: An acid/base reaction. Deprotonation of the oxonium ion reveals the carbonyl C=O in the carboxylic acid product and regenerates the acid catalyst. |
|
| © Dr. Ian Hunt, Department of Chemistry |