| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
Overview of Nucleophilic Acyl Substitution
Overall nucleophilic acyl substitution is most simply represented as follows:
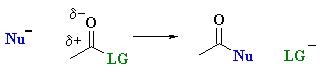
A nucleophile is an electron
rich species that will react with an electron poor species (Nu
in scheme).
An acyl group is R-C=O (where R can
be alkyl or aryl).... note the acyl group in both the starting material and
the product.
A substitution note that the leaving group (LG)
is replaced by the nucleophile (Nu).
There are two fundamental events in a nucleophilic acyl substitution reaction:
| Overall, these events are the same as those in a simple nucleophilic substitution (chapter 8), note the fundamental similarity in the two general processes. | 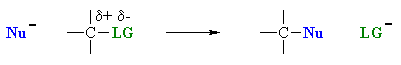 |
The difference in nucleophilic acyl substitution is that when the nucleophile adds to the electrophilic C, it becomes tetrahedral and an intermediate forms, then the leaving group departs as shown below:
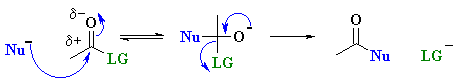
QUESTION:
| © Dr. Ian Hunt, Department of Chemistry |