| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution |
Reduction of Amides
(for more detail see Chapter 22)
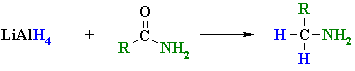
Reaction type: Nucleophilic Acyl Substitution then Nucleophilic Addition
Summary
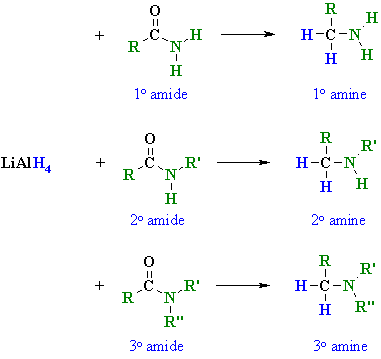
|
|
|
| Step 1: The nucleophilic H from the hydride reagent adds to the electrophilic C in the polar carbonyl group of the ester. Electrons from the C=O move to the electronegative O creating the tetrahedral intermediate, a metal alkoxide complex. |
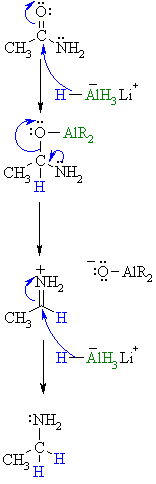 |
| Step 2: The tetrahedral intermediate collapses and displaces the O as part of a metal alkoxide leaving group, this produces a highly reactive iminium ion an intermediate. |
|
| Step 3: Rapid reduction by the nucleophilic H from the hydride reagent as it adds to the electrophilic C in the iminium system. π electrons from the C=N move to the cationic N neutralising the charge creating the amine product. |
|
| © Dr. Ian Hunt, Department of Chemistry |