| Chapter 22: Amines |
| Chapter 22: Amines |
| Qu1: | |
| (a)
|
Use the Lewis
base definition to start and try to look at the lone pair availability
(more available = stronger base). Each system in N centered, so we
can ignore that as a factor. |
| (b)
|
Amines are stronger bases than alcohols. Again we can use lone pair availability.... N is less electronegative than O so it is a better electron donor. What about the alcohol and the thiol ? Acidity increases down a group, so the thiol is a worse base than the alcohol.... larger atoms tend to form weaker bonds with the small proton. |
| (c)
|
Since these are all substituted anilines, we need to look at the role of the substituent (after all, it is the only thing changing across the series). Substituents that are electron donors will make the N lone pair more available (electron donating ability -OCH3 > -CH3) , whereas electron withdrawing groups will make the N lone pair less available (electron withdrawing ability -NO2 > Cl) |
| (d)
|
We can still use lone pair
availability. Piperidine is the most basic (conjugate acid pKa
= 11.2). The lone pair is in an sp3 hybrid orbital
and there is no resonance (no π system). |
| Qu2: | |
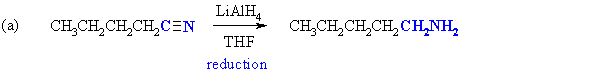
Nitriles, R-C≡N, are reduced to 1o amines by conversion of the R-C≡N to R-CH2-NH2 so the product would 1-aminopentane. |
|
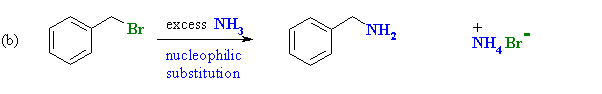
Benzyl bromide is an alkyl halide and will therefore undergo nucleophilic substitution by the nucleophilic N of ammonia giving benzylamine. |
|
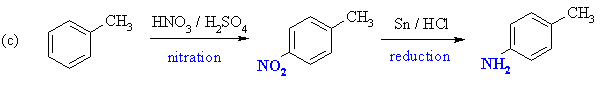
Toluene undergoes nitration to give mainly para-nitrotoluene. Reduction of the nitro group gives the amine, para-aminotoluene (or para-methylaniline if you prefer). |
|
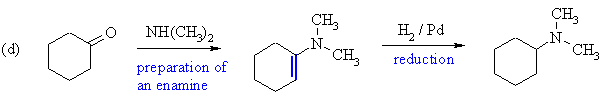
Cyclohexanone, a ketone, will react with a secondary amine to give an enamine (more stable alkene preferred) which on catalytic hydrogenation reduces to the tertiary amine, N,N-dimethylaminocyclohexane. |
|
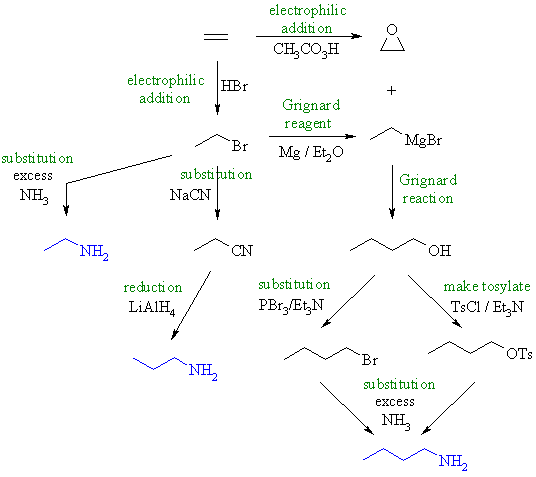
| Qu3: | First, note that we have a homologous series of C2 to C4 amines we are trying to make. |
| Here is a scheme collecting possible syntheses together. These are based on important reactions and to be short syntheses. | |
|
|
|
(a) 1-aminoethane :
we can't add NH3 directly to the alkene, so introduce a halogen,
bromide is a good choice as it is a good leaving group then substitute
using excess ammonia. (b) 1-aminopropane :
we need to add a C atom to get from C2 to C3. For an amine a good way
to do that is via the nitrile. So after preparing ethyl bromide, nucleophilic
substitution with NaCN ( in a polar aprotic solvent such as DMSO) will
give the nitrile which can be reduced to the amine. (c) 1-aminobutane : now we need to add 2C to get to C4.... addition of the Grignard ethyl magnesium bromide to ethylene oxide gives 1-butanol. Ammonia will not react with an alcohol to give substitution (poor leaving group), our best choice here is either to convert the alcohol to the bromide or we could use a tosylate (both better leaving groups) then substitute with the ammonia. |
|
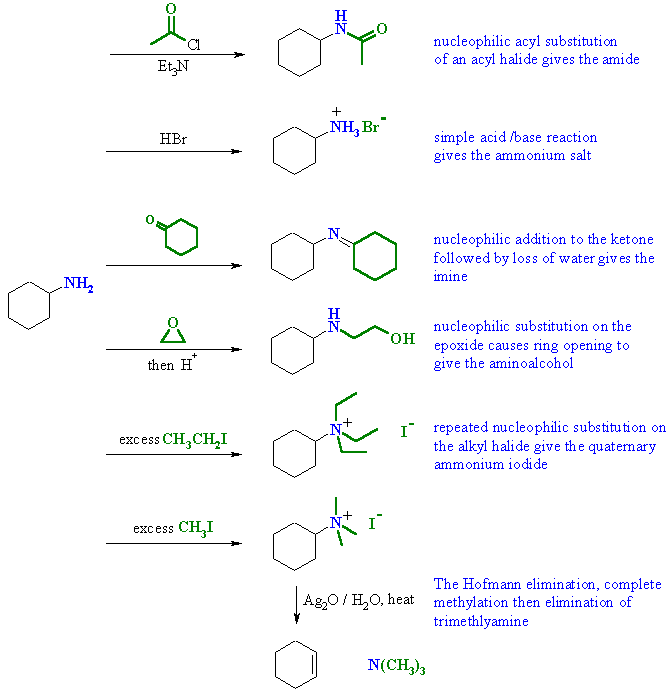
| Qu4: | In each of the reactions the primary amine is the nucleophilic species reacting with a variety of electrophiles..... |
|
|
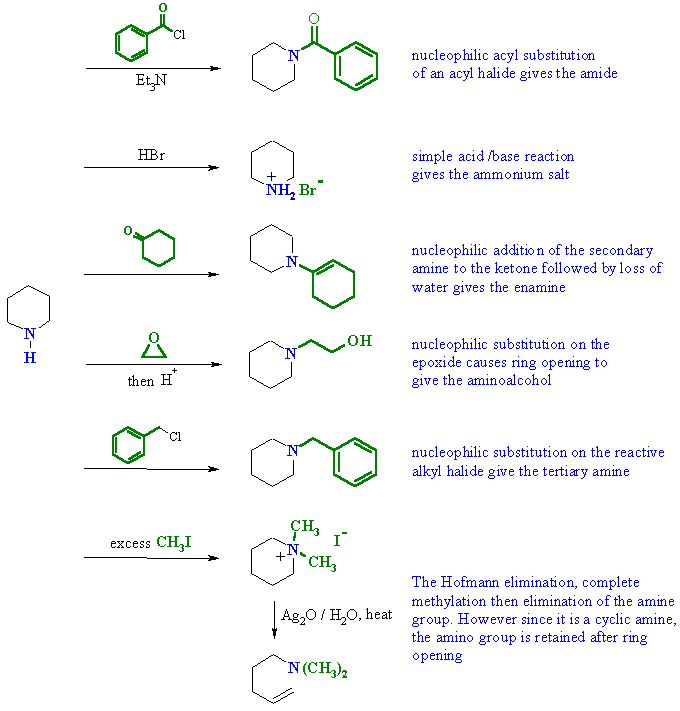
| Qu5: | In each of the reactions the secondary amine is the nucleophilic species reacting with a variety of electrophiles..... |
|
|
| Qu6: | These examples reveal some of the interesting aspects involving amines and aromatic compounds...... |
| (a) |
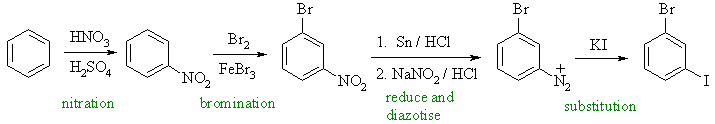
In order to get the two ortho- / para- directing halogens meta- to each other we need to utilise the chemistry of diazonium salts by introducing the first halogen at the nitro stage. This route is especially useful for getting the iodide substitutent. |
| (b) |
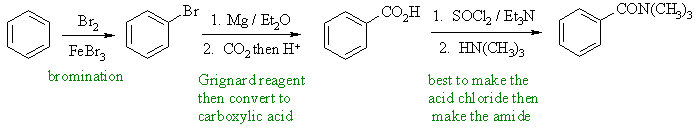
To prepare an amide we need to plan to get the carboxylic acid and the amine. We can prepare the acid by the reaction of carbon dioxide with the Grignard of bromobenzene. Alternative routes to the acid could be the oxidation of an alkyl benzene prepared by alkylation (not shown). |
| (c) |
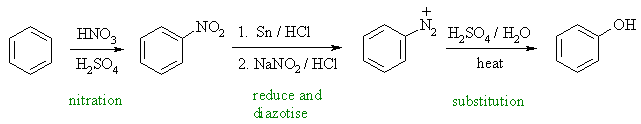
A good route to phenols is via the hydrolysis of the diazonium salt prepared via the conversion of the nitro group to aniline. |
| (d) |
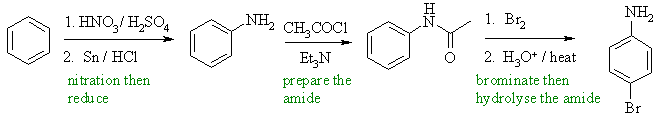
In order to limit the reaction to monobromination, we first prepare the less reactive amide, then brominate, then hydrolyse the amide to the required aniline. |