| Chapter 23: Aryl Halides |
| Chapter 23: Aryl Halides |
Nomenclature:
Functional group suffix = -halobenzene
Functional group prefix = halo-
Numbering of the ring begins at the halogen-substituted carbon and
proceeds in the direction of the next substituted carbon that possesses
the lower number.
Ortho, meta or para ? (review)
Mono-substituted aryl halides are characterised using the prefix ortho
(o-), meta (m-) or para (p-) depending on the placement
of the substituent from the halogen or the halogen from a higher priority
functional group: 1,2-, 1,3- or 1,4- respectively.
|
or o-ethylchlorobenzene |
or m-ethylchlorobenzene |
or p-ethylchlorobenzene |
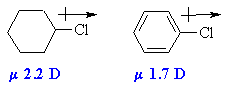
|
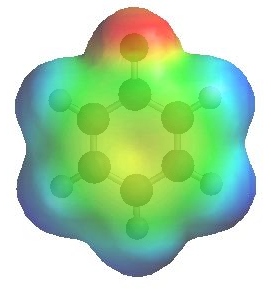
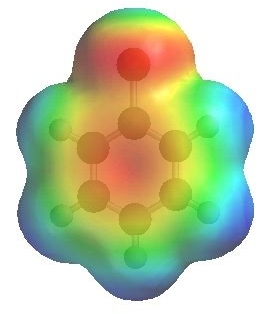
The image to the left shows the electrostatic potential for fluorobenzene
and to the right chlorobenzene
The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
|
|
| © Dr. Ian Hunt, Department of Chemistry |