| Qu1: |
|
(a)
|
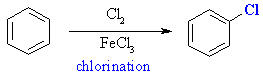
Chlorine atoms can be introduced fairly simply by treating benzene
with chlorine in the presence of a Lewis acid catalyst like iron (III)
chloride.
|
(b)
|
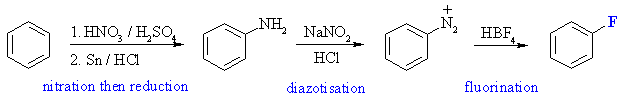
The most common method for introducing fluorine is via the Schiemann
reaction of the diazonium salt using HBF4. Diazonium salts are
obtained from anilines.
|
(c)
|
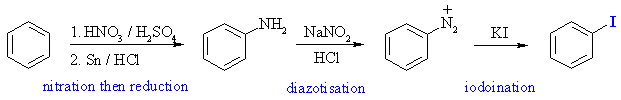
The most common method for introducing iodine is again via the diazonium
salt , by treating it with potassium iodide.
|
| (d)
|
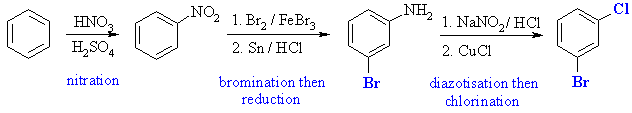
Since both -Cl and -Br are ortho- / para- directing, we cannot
just introduce them using the halogen and iron (III) trihalide method.
The alternative is via the diazonium reactions and a Sandmeyer reaction.
This also means we can use the nitro- group to set up the required meta
arrangement.
|
(e)
|
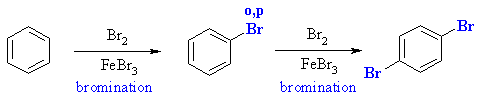
Since bromine is an ortho / para director but the large
size of the bromine to some degree sterically inhibits ortho substitution,
1,4-dibromobenzene can be obtained by treating benzene with excess Br2
and
the Lewis acid catalyst iron (III) bromide.
|
(f)
|
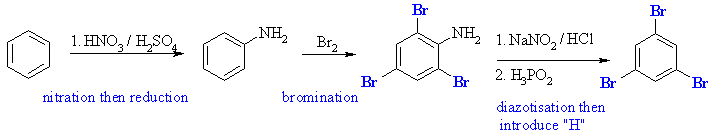
In order to complete this synthesis, we need to use aniline.... this
powerful activating group allows for the introduction of 3 bromines in
the correct arrangement (in fact it is so reactive, a catalyst is not even
needed, and the reaction is almost instantaneous at room temperature),
then the amino group, -NH2, can be removed by diazotisation
and the introduction of a H.
|
|
|
Qu2:
|
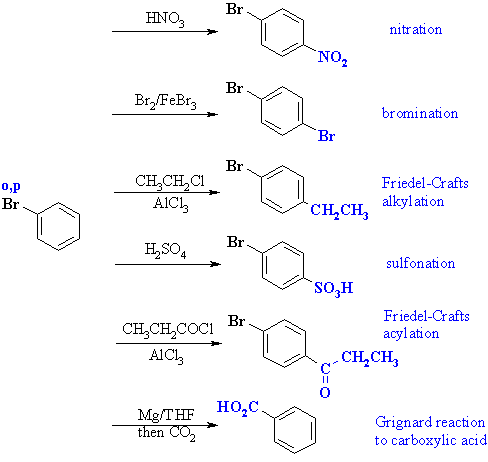
Since the bromine is an ortho- / para- director, but
is fairly large, the major product of these reactions will be the para-
isomers. |
|
|
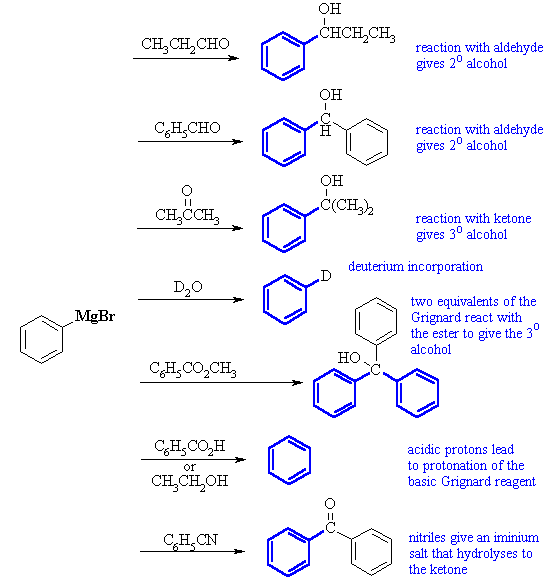
| Qu3: |
A collection of reactions of an aryl Grignard reactions.... you should
compare them with those of ethyl magnesium bromide (see chapter
14 questions). In each case remember that there is a aqueous
acid work-up. |
|
|
|
|
| Qu4: |
Yes benzyne is aromatic..... it has a cyclic, planar, conjugated, 6πelectron system.
|
|
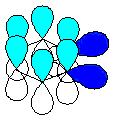 Note that
the second p bond (shown as the blue orbitals
in the diagram to the right) of the triple bond is perpendicular and therefore
cannot overlap with the aromatic π system (shown
as cyan orbitals). This means that the two electrons associated with this
bond are not part of the conjugated system. Note that
the second p bond (shown as the blue orbitals
in the diagram to the right) of the triple bond is perpendicular and therefore
cannot overlap with the aromatic π system (shown
as cyan orbitals). This means that the two electrons associated with this
bond are not part of the conjugated system.
Benzyne is very reactive due to the strain of the triple bond due to
its incorporation into the six membered ring.
|
|
|
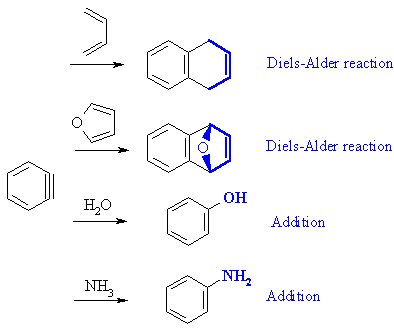
| Qu5: |
Benzyne has a reactive triple bond undergoing addition reactions, including
the Diels-Alder reaction: |
|
|
|
|