| Useful Concepts |
| Useful Concepts |
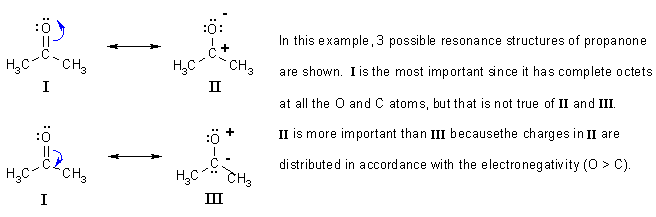
Resonance is probably the single most important concept in organic chemistry since if resonance is present, then it typically controls the outcome.
One of the key applications is understanding substituent effects, particularly in aromatic systems.
The best way to "derive" resonance structures is by learning to "push" curly arrows and starting from a reasonable Lewis structure.
Remember that resonance is a property of π systems. We need to use resonance structures when there is more than one Lewis structure that can present the structure.
Rules to remember for recognising resonance structures:
| © Dr. Ian Hunt, Department of Chemistry |