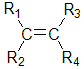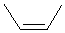 |
Introductory IUPAC Organic
Nomenclature
|
Cis-
and trans- terminology
If alkenes have two different substituents
at each end of the C=C then they can exist as stereoisomers (as geometric
isomers ).
This is because there is restricted rotation of the double bond due to the pi
bond.
For example:
- all terminal alkenes
i.e. those with a C=CH2 unit do
not exist as cis- and trans- isomers.
- all 1,1-symmetrically
disubstituted alkenes i.e. those
with a C=CR2 unit do not
exist as cis- and trans- isomers.
- alkenes with the R-CH=CH-R
unit can exist as cis-
and trans- isomers.
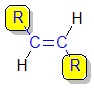
If we consider the general
alkene unit shown below, then the alkene can
only exist as cis and trans isomers if R1is not equal to
R2 AND R3 is not equal to R4.
There are two ways to name these types of isomers, one is the cis / trans method
described here, the other is E/Z and it's described
on the next page.
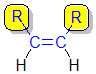
1,2-disubstituted alkenes are described
as:
- cis-
if the two alkyl groups, R-, are on the same side of the C=C
- trans-
if the two alkyl groups, R-, are on the opposite side of the C=C.
- These terms are used
as prefixes.
|
 |
cis-
|
trans- |
For
example, 2-butene, where both R = methyl :
| trans-2-butene |

|
|
| cis-2-butene |
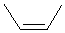
|
|
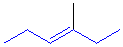
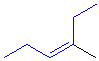
Tri-
or tetrasubstituted alkenes are described as cis- and trans-
based on the relative arrangment of the groups that form the parent hydrocarbon carbon chain that gives the root name. In the example shown the below, the longest carbon chain that gives the root name is highlighted in blue:
|
|
|
trans-3-methyl-3-hexene
|
|
cis-3-methyl-3-hexene
|