 |
Introductory IUPAC Organic Nomenclature
|
 |
Introductory IUPAC Organic Nomenclature
|
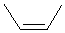 |
 |
| cis-2-butene or Z-2-butene |
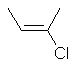
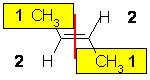
cis-2-chloro-2-butene
or E-2-chloro-2-butene |
 |
 |
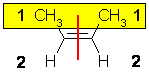 |
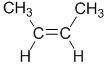
| Step 1 : split the alkene |
Step 2 : assign the relative priorities |
Step 3 : look at the relative positions of
the higher priority groups : same side = Z, hence (Z)-2-butene. |
 |
|
| The two substituents are -CH3 and H, so since the atomic numbers C > H then the -CH3 group is higher priority. Therefore the two high priority groups are on the opposite side, then this is (E)-2-butene. | |
Subrules:
| ©Dr. Ian Hunt, Department of Chemistry |