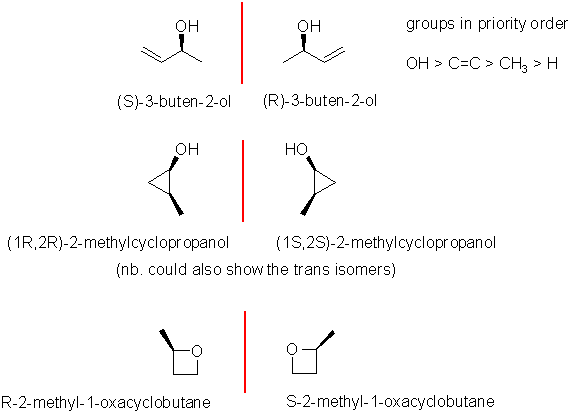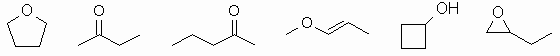
EA:
Since the data doesn't adds up to 100% there must be another element.
IT CAN'T BE A HYDROCARBON !
Prerequisite knowledge should indicate that this is probably oxygen.
But we don't need to
know yet because we have the molecular weight :
%C = 66.63 : 66.63 %
of 72.107 = 48.044
which means there are 48.044 / 12.011 = 4 carbons
%H = 11.18 : 11.18 % of 72.107 = 8.061 which means
there are 8.061 / 1.008 = 8 hydrogens
If we use that we get that C : H ratio
is 1 : 2 and that the %
account for C4H8. That leaves 72.107 - ( 4
x 12.011 + 8 x 1.008) = 15.999 which exactly
matches the atomic weight of oxygen.
Remember NEVER TO ROUND DATA during EA
calculations (it will
invariably mean you get the wrong answer) AND if you add up the MW
using accurate atomic weights it should match exactly.
The molecular formula = C4H8O.
So what functional groups could we have
? The formula is
unsaturated, so we need to remember double bonds too.
We can have the following functional groups : alkene, alcohol,
aldehyde, cycloalkane, epoxide, ether, ketone (for examples, see
diagram below).
Too many students failed to
remember the basic rules of
valence a drew O and C atoms that violated the octet rule by having too
many bonds.
Constitutional isomers have different
connectivities due to
different functional groups or branching. There are lots of
possibilities. Only a few are shown. Biggest error was students
not checking the molecular formula and not even drawing a pair of
isomers !
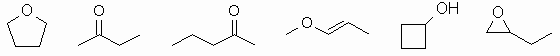
Enantiomers are non-superimposable
mirror images, for that they need
a chirality center, which is this case needs to be an sp3 C
with 4 different substituents attached. There are only a few
possibilities for C4H8O. The biggest error was
drawing that lacked a chirality center or didn't name them. The most
common answer
was the butenol system. In this case you need to make sure you number
it
for the higher priority -OH as an alcohol and remember for name to be
complete you must assign as R
or S.