b.
Index
of hydrogen deficiency ? Either draw out an example and count the
pi
bonds and rings, or use the
formula....
IHD = 1
c.
So what functional groups could we have
? The formula is
unsaturated (IHD > 0) so we can have double bonds or rings too in
the structures.....
We could
have the following functional groups :
Many students failed to
remember the basic rules of
valence a drew O and C atoms that violated the octet rule by having too
many bonds.
Constitutional isomers have different
connectivities due to
different functional groups or branching. The biggest errors were
students
not checking the molecular formula, and not even drawing a pair of
isomers or poor nomenclature !

Enantiomers are non-superimposable
mirror images, for that they need
a chirality center, which is this case needs to be an sp3 C
with 4 different substituents attached. The biggest error was
drawing a molecule that lacked a chirality center or didn't show it in
3D (they are stereoisomers after all). Final task was to assign
as R
or S.
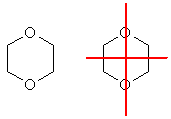
f. The only way to get a compound with the right molecular formula where there is only one type of C, one type of H and one type of O is shown below. The image on the right shows the mirror planes to help you see the symmetry.