Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Not providing justifications for the questions that asked for them.
iv. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.
v. Adding reagents not given in the question and therefore not needed and hence answering a different question!
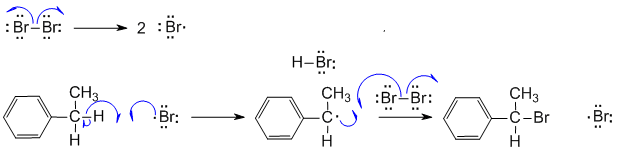
A Radical bromination at the benzylic position (which gives the most stable radical, secondary with resonance). The scheme below shows the initiation and propagation steps:

B The allylic halide undergoes an SN1 reaction to form the alcohol of the secondary carbocation resonance contributor:
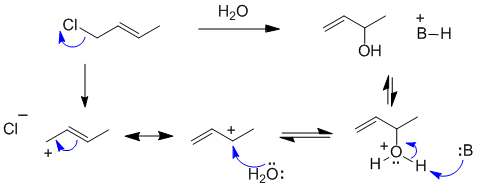
B: = chloride ion or alkene or O functional groups
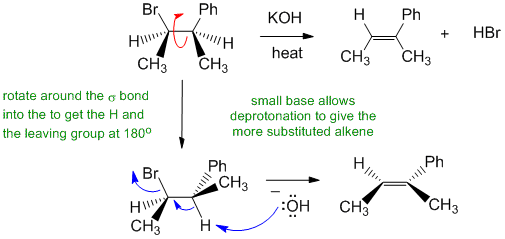
C Alkyl halides undergo elimination via an E2 pathyway when heated with a strong base:
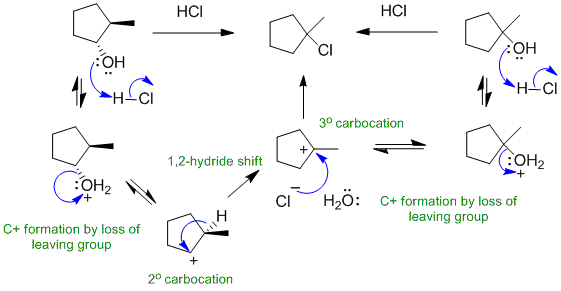
D Alcohols undergo SN1 substitution when reacted with HCl: