| Qu1: |
All are C bonds but there are different bond orders single bonds are the longest, triple bonds the shortest. (v1=B, v2 =C) |
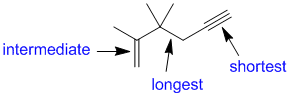 |
| Qu 2: |
Assign the formal charges...N with 2 bonds and 2 lone pairs is -1, N with 3 bonds and a lone pair is 0, and N with 4 bonds is +1 (v1=C, v2=E) |
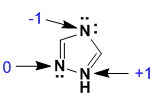 |
| Qu 3: |
Basicity...Think about the availability of the electrons in the bases, always a good idea to draw in the lone pairs because lone pairs are more available than bonded pairs. Ammonium salts are not basic (no available electrons) and the lone pair on an sp3 N is held less tightly (more available) than the lone pair on an sp C. (v1=D, v2 = C) |
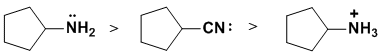 |
Qu 4: |
Increased branching = increased stability (due to the presence of more stronger primary C-H bonds). (v1= AB, v2 =A) |
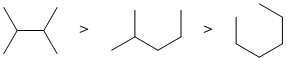 |
| Qu 5: |
The more stable radical can the associated with breaking the weaker C-H to form the radical. So since an sp3 C-H is weaker than sp2 C-H, the vinyl radical is the least stable. The allylic radical is also stablised by resonance. (v1= A, v2 =D) |
|
| Qu 6: |
Counting types of H (models lab). Each type of H will generate a different monochlorination product. Methylcyclopentane has 4 H types, 1,2-dimethylcyclobutane has 3 and cyclohexane has 1. (v1=A, v2=D) |
|
| Qu 7: |
Acidity...the structures are an alcohol, an alkene and a ketone. This means we have an O-H and two C-H systems. The alcohol is the most acidic due to the H on the electronegative O atom (pKa about 15). The ketone is more acidic that the allylic position of the alkene because the H adjacent to the C=O give a carbanion that is resonance delocalised to allow the negative charge on to the electronegative oxygen atom (pKa about 20) which is more acidic than the alkene where the most acidic H will be those in the methyl group where again there will be resonance delocalisation of the carbanion, but only to other carbon atoms (pKa about 45). (v1=B, v2=A) |
|
| Qu 8: |
Ranking resonance structures...we need to check for complete octets and maximised bonding (within the octet rule limitations) and for charge separation in accord with electronegativity. (v1=A, v2=B) |
|
MOLECULAR PROPERTIES:
No real method here, really just do you know various aspects of molecular structure and apply it to the molecule(s) in question. |
| Qu9: |
Acidity...the H on the more electronegative O atoms are more likely to be more acidic. O15 is a phenol (pKa about 10) and O24 a tertiary alcohol (around 15). Thus the phenol is the more acidic (due to the resonance stabilisation in the conjugate base). |
| Qu10: |
N20 is part of a tertiary amine. There is no adjacent pi system so the N is not involved in resonance so the N is sp3 hybridised with the lone pair in an sp3 hybrid orbital. |
| Qu11: |
O7 is in ketone (sp2 hybridisation) and O15 is in a phenol (hence lone pairs are involved in resonance with the pi system and the O is also sp2). |
| Qu12: |
IHD ... Carefully work out MF = C19H21NO4 then use the equation to evaluate IHD = 10 (or, more easily counting from the structure there are 5 pi bonds and 5 rings, 5+5 =10 |
| Qu13: |
O8 is part of an ether, N20 part of an amine. |
| Qu14: |
Assign the R/S stereochemistry, using the Cahn-Ingold-Prelog rules to assign group priorities because the priorty order of attached groups : O8 >C1 (OOC) > C5 (CCC) and H...and when viewing with the H (low priority away), the sense is clockwise => R. The alkene C22-C23 is at the end of the chain and thus is not E or Z. (v1=E, v2=AB) |
| Qu15: |
Bond length will be affected by atom type, hybridisation and bond order. The alkene C22-C23 C=C will be the shortest. (v1=E, v2=D) |
| Qu16: |
NaOH (pKa = 14) is a strong enough base to deprotonate a phenol (pKa = 10) and about the same as a regular alcohol (hence partial deprotonation). (v1=B, v2=D) |
| Qu17: |
C16 is attached to 2 other C atoms and is therefore secondary and it is adjacent to a benzene ring so it is also benzylic. |
SPECTROSCOPY:
Use the IR spectra provided to get the functional groups that are present in the structures, so look for the key groups : C=O (near 1700 cm-1) , -OH or -NH (above 3000 cm-1), aromatic C=C (two bands 1600-1400 cm-1), C-O (near 1200 cm-1) and triple bonds (near 2200 cm-1).... use the tables as needed. |
| Qu18: |
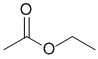
The IR shows a C=O a little above 1700 cm-1. The IR does not show -OH or -NH, or C=C, or triple bonds, but maybe a C-O near 1200 cm-1. This would be consistent with the ester. (v1=BC, v2=E) |
 |
| Qu19: |
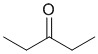
The IR shows a C=O a little above 1700 cm-1. The IR does not show -OH or -NH, or C=C, or triple bonds, and no C-O. This would be consistent with the ketone. (v1=AE, v2=D) |
 |
| Qu20: |
The IR shows does not have a C=O near 1700 cm-1 but does show an -OH near 3300 cm-1. The IR does not show C=C, or triple bonds. This would be consistent with the alcohol. (v1=AB, v2=A) |
 |
| Qu21: |
The IR shows does not have a C=O near 1700 cm-1, nor -OH or -NH, or C=C. There is an absorption at 2100 cm-1 that indicates a triple bond and the peak at 3300 cm-1 would be consistent with a terminal alkyne. (v1=CD, v2=AB) |
 |
| Qu22: |
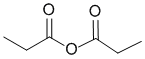
The IR shows two absorptions above 1700 cm-1 that look like C=O. The IR does not show -OH or -NH, or C=C, or triple bonds. This would be consistent with the anhydride (sym and asym C=O stretches. (v1=AD, v2=C) |
 |
| Qu23: |
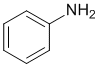
The IR shows does not have a C=O near 1700 cm-1 but does show an -NH2 near 3300 cm-1 (2 bands). The IR also shows C=C (near 1600 and 1500 cm-1, and the C-H are just above 3000 cm-1 indicating sp2 C-H. This would be consistent with the aromatic amine. (v1=C, v2=AE) |
 |
NOMENCLATURE:
Two types of questions here, however it is a good idea to use key descriptors like E and Z, R or S, cis or trans, numbering, functional group etc. to help screen the answers before getting too tied up in the words. For the first four, name the compound and then match it to the list, for the last four
use the key descriptors and check each molecule in turn. So this section really depends on the nomenclature rules. |
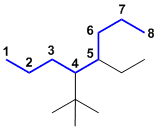
| Qu24: |
An alkane with the longest chain C8, so an octane. There are two substituents a simple ethyl group and a complex group. Complex groups are alphabetised based on the first letter in the brackets. In this example, the alphabetical order of the substituents dictates the numbering. Hence, 4-(1,1-dimethylethyl)-5-ethyloctane. (v1=B, v2=C) |
 |
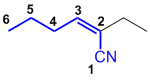
| Qu25: |
The longest chain including the alkene and the nitrile is C6 so a hexenenitrile. With the C in the nitrile as C1, the alkene is C2-C3 with an ethyl group at C2. The double bond has stereochemistry, so the Cahn-Ingold-Prelog priority rules need to be applied, CN > ethyl and alkyl > H thus a Z system : (Z)-2-ethylhex-2-enenitrile. (v1=A, v2=B) |
 |
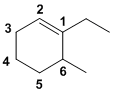
| Qu26: |
The ring containing the alkene is C6 therefore a cyclohexene. The alkene defines the numbering, with the first point of difference requiring that we define the alkene C with the ethyl group attached as C1. Since we must number via the alkene, this means the methyl group is at C6. Hence, 1-ethyl-6-methylcyclohexene. (v1=E, v2=AB) |
 |
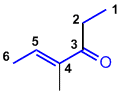
| Qu27: |
The longest chain including the alkene and the ketone is C6, so we have a hexenone. Numbering to give the higher priority functional group the lowest possible number, we have a hex-4-en-3-one. There is a methyl group at C4 and the double bond is trans based on the shape of the hexenone chain : trans-4-methylhex-4-en-3-one. (v1=B, v2=E) |
 |
| Qu28: |
Naming of simple alkyl groups (v1=C, v2=D) |
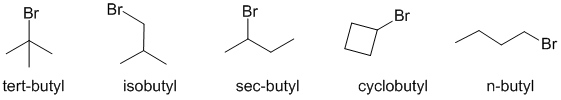 |
| Qu29: |
Substituted benzenes and ester naming : (v1=C, v2=D) |
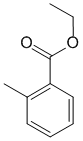 |
| Qu30: |
R/S stereochemistry
Chirality center is marked with a *. Priority of groups there is OH > C(CCC) > C(CCH) > H. (v1=B, v2=A) |
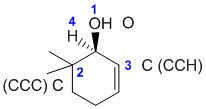 |
| Qu31: |
The bicyclic system has 8C and an alkene so it is an octene. The [3.2.1] indicates the number of C atoms in each link between the bridgehead carbons. Need to start to number from a bridgehead, going along the longer link first to give the alkene the lowest number and hence locate the methyl group (v1=C, v2=D) |
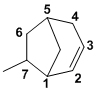 |















