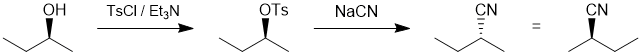Chem 351 Final Fall 2022
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
| Qu 1: |
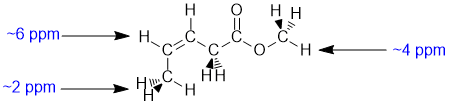
Chemical shifts of the groups in question in these systems are determined by the position of the H (that is what is changing). Chemical shifts are affected by electronegative atoms (especially such as O) and magnetic anisotropy (due to pi systems). The vinyl H (C=C-H 5-7 ppm) is deshielded by the magnetic anisotropy of the alkene unit. The methyl -O-CH3 group is affected by the electronegativity O that it is directly attached to (about 4 ppm) and the allylic methyl CH3-C=O is affected the proximity of the alkene unit (about 2 ppm). |
 |
| Qu 2: |
Acidity.... Need to look at the stability of the conjugate bases and consider the factors that affect that stability. We have CH, OH and SH systems but the O is +ve, the other two are neutral. The pKa of the oxonium system (O+) will be like H3O+ = 0, the thiol (RSH) is about 10 while allylic methylene is likely about 40 (remember a lower pKa implies a stronger acid). |
 |
| Qu 3: |
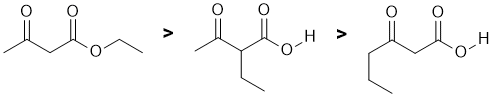
This is really about acidity.... NaNH2 is a strong base and we need to look at the stability of the conjugate bases and consider the factors that affect that stability. We have a carboxylic acid (pKa = 5), and then a pair of positions that are alpha to C=O groups, but one is between two C=O ("active methylene" pKa about 11) and the other only adjacent to a ketone (pKa = 20). Remember a lower pKa implies a stronger acid. The base is more likely to deprotonate the more acidic H creating the nucleophile that then undergoes SN2 with the ethyl bromide. |
 |
Qu 4: |
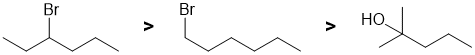
First identify the reaction...dehydrohalogenation of alkyl halides. Draw the starting material and the products, then consider the alkenes based on 1,2-elimination following Zaitsev's rule (formation of the more stable, more highly substituted alkene, nature of the leaving group (remember that alcohols require acid to form a good LG and hence simple alcohols don't eliminate under these conditions). Note that in E2 reactions, the relative reactivity order of alkyl halides is tertiary > secondary > primary. |
 |
Qu 5: |
This is a radical substitution reaction of alkanes (i.e. sp3 C-H bonds). For bromination, the reaction is essentially controlled the relative reactivity of the radical formed and the number of H that yield that radical. The relative reactivity of tertiary : secondary : primary systems are 1640 : 82 : 1 (which means that the number of H at each position is usually irrelevant). Note that the benzylic secondary radical is more stable than a simple secondary radical. |
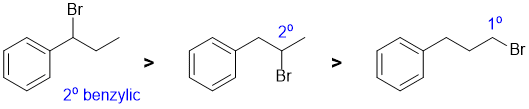 |
Qu 6: |
The number of lines = multiplicity (i.e. coupling) of the signals for each of the positions indicated is determined by the number of neighbours (n) that are of a different type and the n+1 rule. Here we see a singlet (0 neighbours), a doublet (1 neighbours) and a quartet (3 neighbours). |
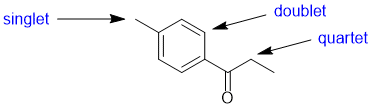 |
Qu 7: |
Basicity.... the strongest base will produce the most the greatest amount of the conjugate base. The strongest base is the amide ion (associated pKa = 35) then hydroxide (pKa approx. 15) and lastly the alcohol (pKa about 0).... for basicity, think about where the e are coming from ? -ve more available than neutral and the electronegativity of the atom (same row) carrying the lone pairs will affect electron availability. |
NaN(i-Pr)2 > NaOH > CH3OH |
| Qu 8: |
First identify the reaction...dehydrohalogenation of alkyl halides and we should be thinking about Zaitsev's rule. Increasing the bulk of the base (sterics) tends to increase the amount of the more accessible H that is removed (i.e. favours the anti-Zaitsev product). The metal ion is a spectator and therefore essentially irrelevant. |
(CH3)3O- > (CH3)2CHO- > CH3CH2O- |
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
| Qu9: |
The amide will be favoured since the N is more nucleophilic than the O (note that this relates to the synthesis of acetaminophen laboratory experiment). |
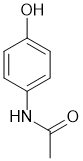 |
| Qu10: |
The reaction of alcohols with HBr tends to be SN1 via C+... aromatic alcohols (phenols) do not for stable C+ under these conditions so are unreactive in SN1, while benzyl alcohols form a favourable resonance stabilised C+. |
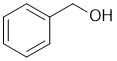 |
| Qu11: |
Cahn-Ingold-prelog rules are based on the heavier atom at the first point of difference which would the F vs a C (the Br is too far away and hence irrelevant) |
-CH2F |
| Qu12: |
Acidity... pKas help.... carboxylic acids have a pKa about 5, ammonium salts about 10 and oxonium about 0. So at pH = 7, the acid will be deprotonated (i.e. exist as the carboxylate, RCO2-), the ammonium will not (i.e. it will exist as the RNH3+) and the oxonium will be deprotonated (i.e. it will exist as the alcohol, ROH not ROH2+) |
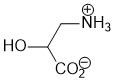 |
| Qu13: |
Pathway I will not give the ether because an acid / base reaction will occur to give methanol and n-propanol while II can and does undergo a nucleophilic substitution reaction (SN2) |
|
| Qu14: |
The best way to "derive" resonance structures is by pushing the arrows... |
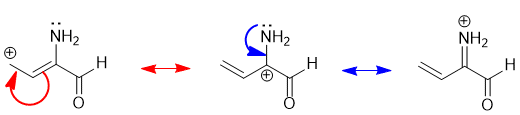 |
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
| Qu15: |
We should work forwards... the first reaction is an SN2 involving the conversion of the alcohol to the alkyl halide using thionyl chloride via a nucleophilic substitution with inversion of stereochemistry followed by a second SN2 of the alkyl chloride to an alkyl iodide with a second inversion (so back to the original trans-stereochemistry). (D) |
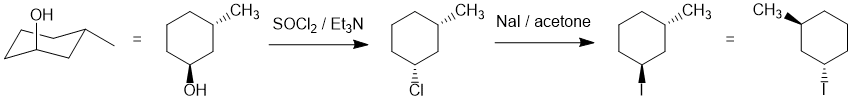 |
| Qu16: |
We should work forwards... since we are starting with an alcohol and are treating it with aq. acid at room temperature, it is expected to protonate to make the better leaving then loss of water will give a secondary carbobcation (i.e. SN1). Since that secondary C+ is next to a branch point, it undergoes a 1,2-hydride shift (a carbocation rearrangement) to give a more stable tertiary C+. With the aq. conditions, a molecule of water is going to be the nucleophile which means we end up with the rearranged alcohol. The reaction conditions (aqueous and at room temperature) are not likely to result in an elimination (dehydration). (C) |
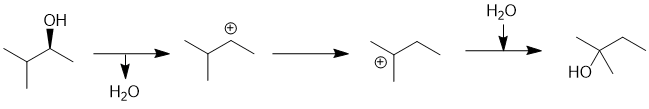 |
| Qu17: |
We should work forwards...but starting with a review of the overall transformation by comparing starting material and product. Since we are starting with an alcohol and forming an alcohol, we are doing an elimination. We should note that it is then an anti-Zaitsev elimination giving the less highly substituted exocyclic alkene while simple alcohol dehydration tends to be Zaitsev (giving the more highly substituted alkene). This means we need to do an E2
elimination and use of the bulky base and having first converted the -OH into a different leaving group. (D) |
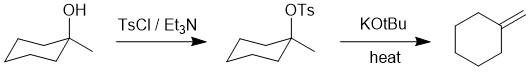 |
| Qu18: |
We should work forwards...but starting with a review of the overall transformation by comparing starting material and product. Both the starting material and the product are methyl esters but the product has gained +1C, on the carbonyl side... so that suggests the formation and then alkylation of an enolate. This would require a strong enough base (ester enolate pKa = 25) and then likely a methyl halide. (E)
|
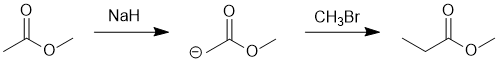 |
| Qu19: |
We should work backwards... the product is an alkene and it's being created by heating with base... this suggests an elimination of an alkyl halide. When considering E2 reactions on rings systems, the stereochemical requirement for the H-C-C-LG to be anti (180 degrees) needs to be considered and this will likely be the factor that ensures the single product in this question. In order for that to be the case, the Br will need to be between the 1,3-alkyl groups with the -Br and -Et trans but the -Br and -Me cis. Remember that on a cyclohexane, for the -Br to eliminate it will need to be axial, and it that conformation, if the -Et is also axial it will ensure the correct regiochemistry. (E)
|
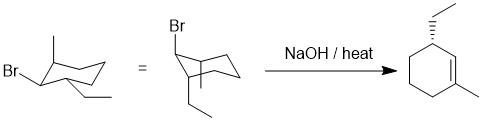
|
| Qu20: |
We should work forwards... the first reaction the conversion of the alcohol to the tosylate using tosyl chloride and then a nucleophilic substitution (SN2) of the tosylate with cyanide as the nucleophile. The SN2 will cause an inversion of stereochemistry, so we need the nitrile with the opposite configuration to that at the -OH center in the starting material. (C) |
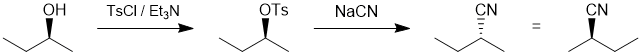 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
Qu 21:
The two Cl atoms are at 60 degrees (torsional angle) to each other which is a gauche conformation. (D)
Qu 22:
The two structures have the same groups (e.g. two methyl and two ethyl groups) connected in the same order and only differ by rotation about a single bond, hence they are conformational isomers (C)
Qu 23:
During a ring flip, anything axial goes equatorial and vice versa and of course the relative position (e.g. 1,2- of the substituents does not change). (D)
Qu24:
First draw out the named structure in a favoured chair conformation. Trans- means that the ethyl and methyl groups are on opposite faces of the cyclic system and being 1,3- this means one axial and one equatorial. Therefore the lowest energy conformation with have the larger ethyl group in the more favourable equatorial position. (E)
Qu25:
The easiest approach is to build the model as per the wedge-hash diagram then hold it such that you have the C-F system at the front with the methyl up and the F will be down to the right. This rules out A and B. Then check the position of the twin methyl groups to rule out D and E. This leaves (C)
Qu26:
In order to have a chirality center, the sp3 C will need to have 4 different groups attached (don't forget that there may be an unshown H that you need to assume is present based on the drawing conventions. Neither B (two -OH groups) ) nor C (two -OCH3 groups) have chirality centers. (ADE)
Qu27:
Constitutional isomers have the same set of atoms (C3H7NO3) connected in a different order (e.g. different functional groups and/or substituents). A good place to start is counting N and O atoms... making B and C the only possible candidates but C has 4 C atoms. (B)
Qu28:
Conformational isomers have the same atoms connected in the same order only differ by rotation about a single bond. Only A and D have the same connectivity (note the relative positions of the -NH2 and -OCH3 groups. D has the same conformation as that shown in the question. (A)
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.
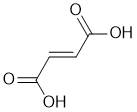
| Qu29: |
IR shows a carbonyl (i.e. C=O) at 1695 cm-1 which is low for a ketone as is the C-NMR peak at 177 ppm. The C-NMR shows only 2 C types one being the C=O (177 ppm) and the other likely C=C (137 ppm). The H-NMR has 2 types of H. The 1H singlet at 6.65 ppm,
suggests a -CH group maybe on C=C and the 1H singlet at 13.0 ppm,
suggests a -CO2H. Remember that one does not observe coupling between H of the same type. |
|
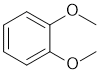
| Qu30: |
IR has no carbonyl (i.e. C=O) near 1715 cm-1 but shows C=C near 1607 cm-1 and likely both sp3 and sp2 C-H 2909 and 3065 cm-1 respectively. The C-NMR shows 4 C types including 3 ArC 110-170 ppm that suggests a substituted benzene. The H-NMR has at least 2 types of H (note that all the ArH at 6.9ppm are showing as a multiplet). The 3H triplet at 3.9 ppm,
is consistent with a -CH3 group attached to something that deshields such as an O. With 3 ArC types, this would match a symmetrically substituted ortho isomer. |
 |
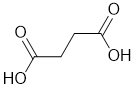
| Qu31: |
IR shows a potential carbonyl (i.e. C=O) at 1693 cm-1 which is low for a ketone as is the C-NMR peak at 173 ppm suggesting a carboxylic acid or derivative. The H-NMR has 2 types of H. The 2H singlet at 2.4 ppm,
suggests a -CH2- group and the 1H singlet at 12.2ppm suggests a -CO2H. Remember that one does not observe coupling between H of the same type. |
 |
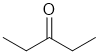
| Qu32: |
IR shows a carbonyl (i.e. C=O) at 1720 cm-1 which is very close to the typical value for a ketone. The C-NMR shows 3 C types including a peak for the C=O at 212 ppm suggests an aldehyde or ketone. The H-NMR has 2 types of H. The 3H triplet at 1.1 ppm,
suggests a -CH3 group next to a CH2, the 2H quartet at 2.4 ppm suggests a CH2 group with 3 neighbours, so it looks like we have a -CH2- group next to a -CH3,. These pieces of data are consistent with a ketone with two ethyl groups. |
 |
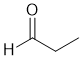
| Qu33: |
IR shows a carbonyl (i.e. C=O) at 1733 cm-1 which is high for a ketone as is the C-NMR peak at 203 ppm which suggests an aldehyde or ketone. The C-NMR shows 3 C types. The H-NMR has 3 types of H. The 3H triplet at 1.1 ppm,
suggests a -CH3 group next to a CH2-. The 2H quartet at 2.5 ppm,
suggests a -CH2- group with 3 H neighbours i.e. an ethyl group. The 1H singlet at 9.8 ppm,
would be consistent with an aldehyde CHO. |
 |
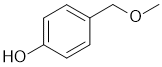
| Qu34: |
IR has no carbonyl (i.e. C=O) near 1715 cm-1 but shows -OH near 3200 cm-1 and broad. The C-NMR shows 6 C types including 4 ArC 110-170 ppm that suggests a substituted benzene. The H-NMR has 5 types of H. The 3H singlet at 3.4 ppm,
is consistent with a -CH3 group attached to something that deshields (e.g. O). The 2H singlet at 4.4 ppm,
is consistent with a -CH2 group attached to something that deshields (e.g. O). The 6.0 ppm 1H broad exchangeable singlet would be an -OH, then two 2H doublets (6.7 and 7.2 ppm) a para disubstituted benzene. |
 |