Part 8: MECHANISMS
Note that no other reagents are
needed in order to complete
any
of these sequences, you should only be using what is there.

IMAGES WILL BE ADDED AS TIME ALLOWS
LAST UPDATE JAN 17th 2006
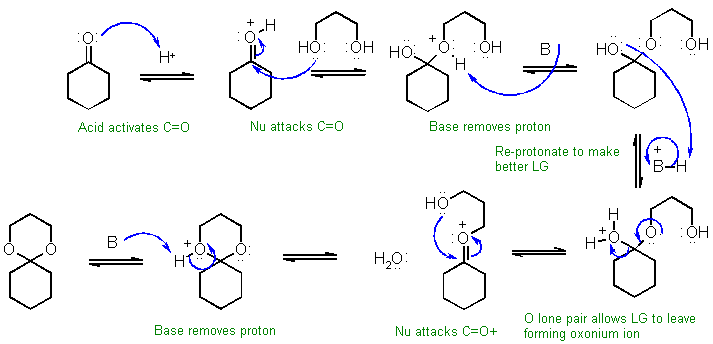
A
Acid catalysed hydrolysis of a cyclic
acetal to the ketone and the
corresponding 1,2-diol. Note the base, B:, could be a water
molecule,
the conjugate base of the acid catalyst, a molecule of the acetal etc. As a temporary solution, it would
be
very like the following....
B
Acid catalysed hydrolysis of an amide
to the parent carboxylic acid
and the corresponding alcohol. Here the benzene part has been
abbreviated
to "Ar". Note the base, B:, could be a water molecule, the
conjugate
base of the acid catalyst or an ester molecule.
For an example see: /courses/351/Carey5th/Ch20/ch20-3-4-1.html
C
Preparation of an epoxide via an
electrophilic addition to an alkene
using a peracid.
For an example see : /courses/351/Carey5th/Ch06/ch6-9.html
D
Friedel-Crafts alkylation of an
aromatic via an electrophilic aromatic
substitution involving a carbocation rearrangement.
For an example see : /courses/351/Carey5th/Ch12/ch12-6.html
![[Chem 350 Home]](mol.gif) Return
to Homepage
Return
to Homepage