Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
Part A:
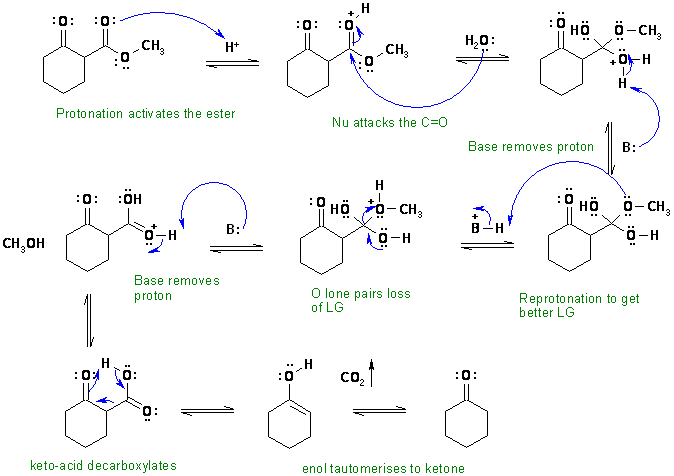
i. Ester hydrolysis followed by decarboxylation of a b-ketoacid.
In this scheme, the base B: could be water, an alcohol, one of the carbonyl
groups or the conjugate base of the acid catalyst.

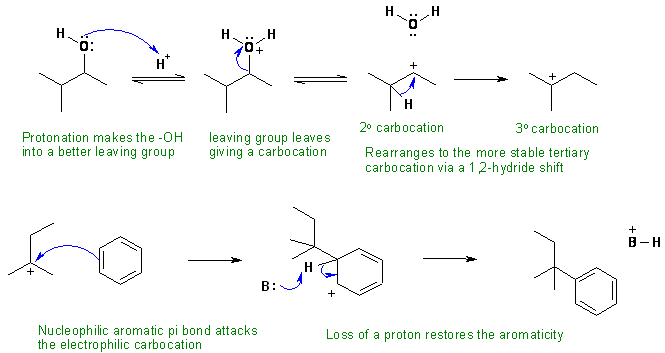
ii. Alkylation of benzene, an electrophilic aromatic substitution via a tertiary carbocation formed by 1,2-hydride shift from a secondary alcohol. In this scheme, the base B: could be water, an alcohol or the conjugate base of the acid catalyst.
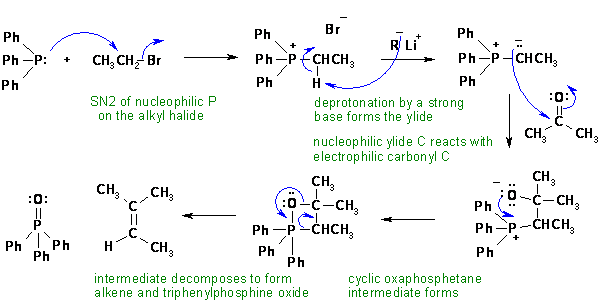
iii. A Wittig reaction.
Part B:
i. The benzoin condensation.... the cyanide reacts with the aldehyde to form a cyanohydrin system (note that the cyanide ion is not basic enough to deprotonate the aldehyde H). The cyanohydrin anion equilibrates to a nitrile enolate which then adds to the other aldehyde, a proton exchange and loss of the nitrile gives the product.
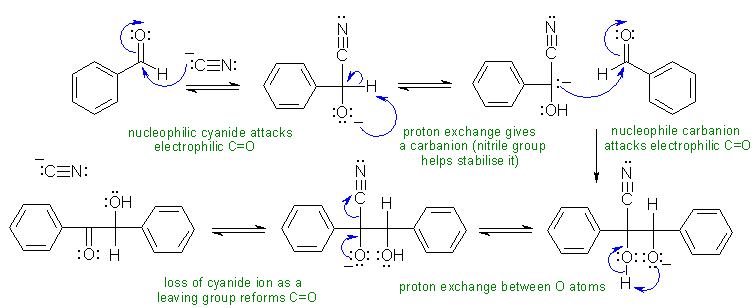
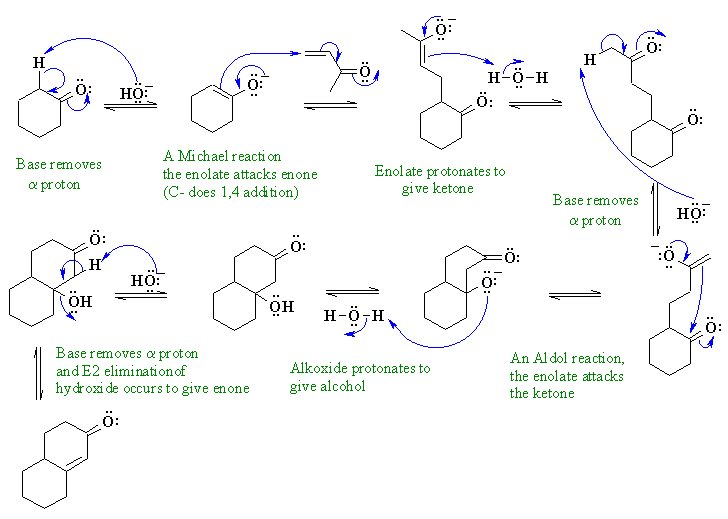
ii The Robinson annulation : Michael addition (a type of conjugate addition) of an enolate on a conjugated ketone followed by an intramolecular aldol reaction then elimination of an alcohol to give a conjugated ketone.