Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting on a bond or lone pair.
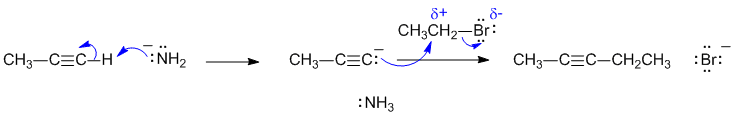
A1
The reaction is that of a terminal alkyne with a strong base to give a carbanion that is a good nucleophile. When this is reacted with an alkyl bromide, the nucleophilic C reacts with the electrophilic C to form a new C-C bond in an SN2 type reaction and hence a new alkyne. This is an alkylation of a terminal alkyne.

Common errors: (1) The process is not a radical reaction. (2) NaNH2 is essentially ionic in that it can be thought of as Na+ and -NH2 . If you consider it as ionic with formal full charges, you can't show a covalent bond. (3) Since the alkyl halide here is primary, it will NOT form a carbocation so the reaction is not an SN1.
A2
The reaction is the hydrolysis of a lipid (fatty acid ester) under basic conditions (this relates and connects the laboratory experiments on the plastic recycling and biodiesel).
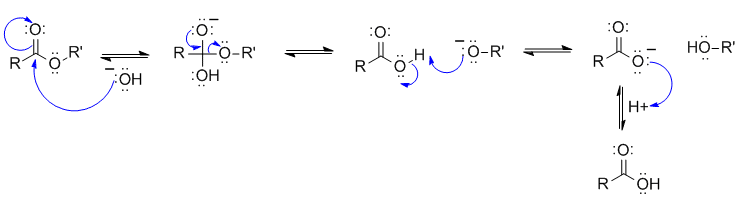
Common errors: (1) not showing the carboxylic acid reacting with the alkoxide (as per step 3 above) ... think about the pKas involved (2) having the HO- attack at the wrong C atom in the initial ester (which C is the most electrophilic?)
B1
The reaction is a methyl Grignard reagent with an epoxide. Grignard reagents are a source of nucleophilic carbon - a strong nucleophile - which opens the epoxide by attacking the least hindered end in an SN2 fashion.
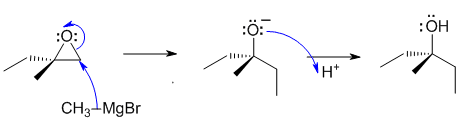
Common errors: (1) wrong nucleophile used (i.e. Br- instead of "CH3-"). (2) Incorrect regiochemistry. (3) wrong functional group in the product. (4) RMgX can be "veiwed" as if it were ionic in that it can be thought of as Mg2+, -CH3 and Br-. If you consider it as ionic with formal full charges, you can't show a covalent bond.
B2
The reaction is an electrophilic addition to an alkene. It is closely related to simple hydration but instead of water being the nucleophile, here it is an alcohol, so instead of the product being an alcohol, it's an ether.
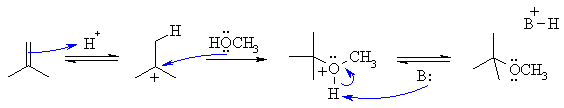
B: could be the conjugate base of the acid catalyst used, or methanol or the alkene
The first step is protonation of the alkene with the H+ to give the more stable carbocation, in this case a 3o carbocation. Then the lone pairs on the O act as the nucleophile attacking the cation. Loss of the proton gives the product. It is important to remember that in an acidic solution (we have H+ defined) that the amount of alkoxide ion (i.e. CH3O-) is minimal.... think of the pKa which for this dissociation would be about 16. So the nucleophile will be the alcohol itself (i.e. CH3OH).
Common errors: (1) Using B: to represent a base in a reaction but then not listing what B: could be based on species that are present in the reaction mixture. (2) Using HO- as the base in an acidic medium (3) Using CH3O- as the nucleophile in an acidic medium (4) saying the base was water when there is no water present1 (read the question!)