Part 5: MECHANISMS
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting where the electrons are i.e. on a bond or lone pair.
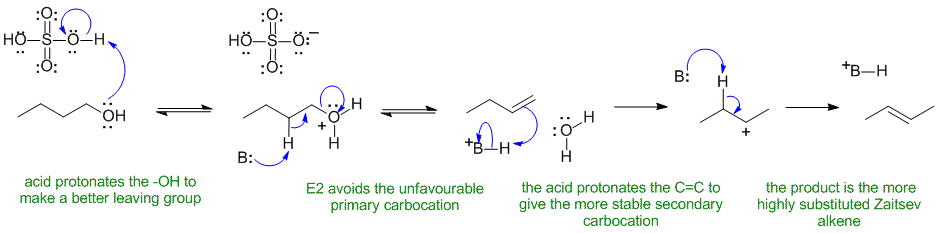
A1
Normally alcohols undergo elimination via an E1 pathyway when heated with a strong acid, but since primary carbocations are so unstable, primary alcohols eliminate via E2 mechanisms:

In this scheme, the base B: could be HSO4-, H2O or the alcohol itself
Common errors:
A2
Base catalysed tautomerisation of an enol to a ketone:
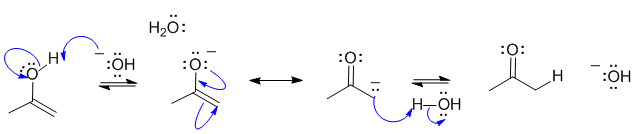
Common errors:
B1
The reaction is an acetylide ion with an epoxide. Acetylide ions are a source of nucleophilic carbon - a strong nucleophile - which opens the epoxide by attacking the least hindered end in an SN2 fashion. Note that the acid (work up) is not added until after the Grignard has been added to the epoxide.
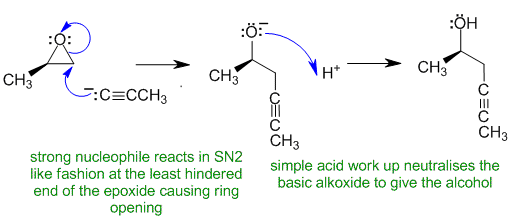
Justification: The acetylide ion is a strong nucleophile (a carbanion) and reacts with the epoxide directly. Due to the strong nucleophile, the reaction is SN2 like and the nucleophile attacks the less sterically hindered C atom which opens the epoxide ring.
Common errors:
B2
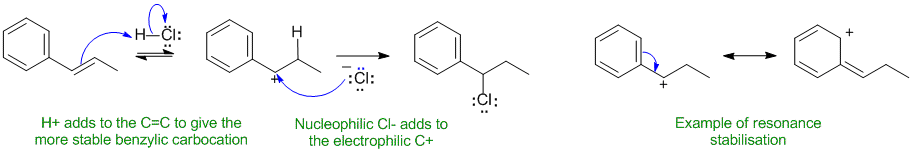
Justification: The alkene reacts with the electrophilic proton to make the more stable carbocation. In this case this will be the carbocation adjacent to the benzene ring, i.e. a benzylic carbocation, because the benzene ring can stabilise the C+ center through resonance.
Common errors: