| Chapter 1: Where are the Electrons ? |
| Chapter 1: Where are the Electrons ? |
Atomic structure
Let's start with a review of some key aspects of atomic structure:
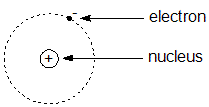 |
* the charge on a proton is 1.602 x 10-19 C |
|
||
|
|
|||
The periodic
table of elements is arranged based on increasing atomic
number, starting with hydrogen at the top left. As we traverse the periodic table, from the top left, going left to right, then down to the next row, at each step we are adding another proton to the nucleus and therefore another electron. Note that when getting the mass number from a
periodic
table, it usually gives the relative atomic mass, a weighted
average
of the isotopic masses based on a normal, natural sample.
Could you
determine how many protons, neutrons or electrons a specific atom or ion has from it's atomic symbol or the periodic table?
In introductory organic chemistry most of the organic compounds we will be interested in are based on only
a small group of elements: H, C, N, O, and the halogens Cl, Br and I. We will also occasionaly encounter
a
few
other atoms such as Li, Na, Mg, Al, B, P, S and F. In more advanced organic chemistry, when you look at
more advanced synthetic methods, one needs to be familiar with many
more
elements.
Try the questions about atoms.
 |
© Dr. Ian Hunt, Department of Chemistry |