 |
Chapter 1: Where are the electrons ? |
 |
Orbital Quantum numbers
From General chemistry, you should know about quantum numbers and what they tell you about and orbital.
The properties of orbitals (energy, size, shape etc.) and the
electrons within the orbitals (location, spin) are described using
quantum
numbers:
| quantum number |
symbol |
value range |
describes
|
|
principal
|
n
|
1, 2, 3, .... |
energy level |
| angular momentum |
l
|
0 to n-1 |
orbital shape (s = 0, p = 1, d = 2 etc.) |
|
magnetic
|
ml
|
- l to + l |
spatial orientation and degeneracy
(number of orbitals of that type) |
|
spin
|
ms
|
± 1/2 |
electron spin |
Given an orbital, the quantum
numbers that describe that orbital can
be determined, or, given a set of quantum numbers one can also
determine
the type of orbital:
|
n
|
l
|
ml
|
orbital |
|
1
|
0
|
0
|
1s
|
|
2
|
0
|
0
|
2s
|
|
1
|
-1
|
2px
|
|
0
|
2py
|
|
+1
|
2pz
|
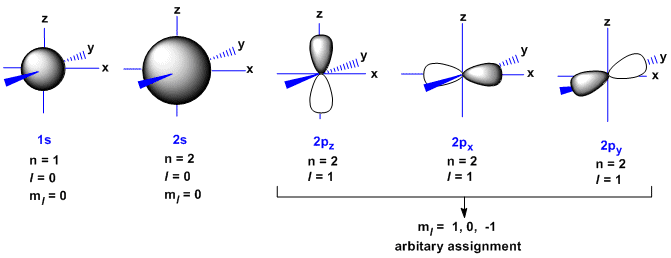
The orbitals for the electrons in carbon, and the necessary
quantum numbers are given below. The nucleus of the carbon atom
would
reside
at the origin of the x, y, z axes.
Try some questions.