| Chapter 2: Bonding |
| Chapter 2: Bonding |
Bonding in "He2"
Why can't helium form bimolecular helium molecules (He2)
analogous to H2 ?
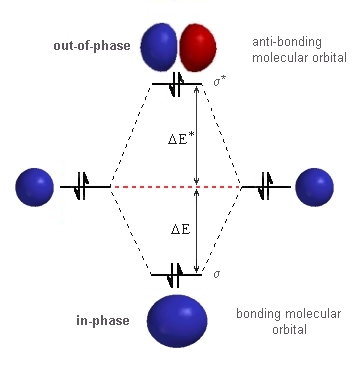
| Good news ! We can use the same basic molecular orbital diagram that
we used for hydrogen because the starting point is the same set of atomic orbitals, the 1s orbitals. The only difference is that the number of electrons involved. Each 1s orbital now contains a pair of electrons.
Everything is the same until we consider
in the electrons. The stabilisation due to the electrons in the σ-bonding orbital is given by 2 DE. The destabilisation due to the electrons in the σ* -anti-bonding orbital is give by 2 DE*. The critical factor from the quantum mechanical treatment is that the magnitude of DE* > DE. Hence, the destabilisation due to the electrons in
the σ*-anti-bonding orbital is greater than the stabilisation
due to the electrons in the σ-bonding orbital : the net effect being there is more destabilisation than there is stabilisation. Therefore, a molecule of
He2 is less stable than 2 atoms of He ! |
 |
||
|
Conclusion
 |
© Dr. Ian Hunt, Department of Chemistry |