| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
When a C atom is attached to 2 groups and so is involved
in 2 π bonds, it requires 2 orbitals in the hybrid set. This requires that
it is sp hybridised. The general "steps" are similar to that for seen
previously sp3 and sp2 hybridisation.
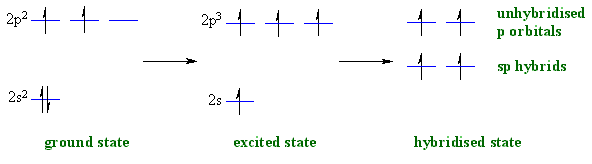 |
|
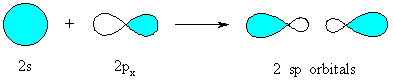
(note the sp hybrids are offset horizontally to allow both lobes to be seen) |
|
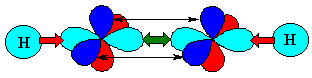
You can view an animation of the hybridisation of the C orbitals if you wish. |
|
|
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |