| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
Summary:
| Functional group suffix =
-ane Functional group prefix = alkyl- |
e.g. CH3CH3
= ethane e.g. CH3CH2- = ethyl |
More details on alkane nomenclature?
Physical
Properties:
The low polarity of all the bonds in alkanes means that the only intermolecular
forces between molecules of alkanes are the very weak induced
dipole
- induced dipole forces. These forces are easily overcome.
As a result, in comparison with other functional groups, alkanes
tend to have low melting and boiling points and very low
solubility
in polar solvents such as water (remember "oil and water don't mix" and
the adage "like dissolves like").

Structure:
Consist of only sp3 hybridised C and H atoms connected by
Ï
bonds.
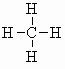
| methane CH4 (b.pt. = -160oC) |
|
|||
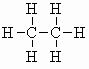
| ethane C2H6 (b.pt. = -89oC) |
|
|||
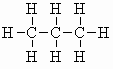
| propane C3H8 (b.pt. = -42oC) |
|
|||
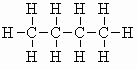
| butane C4H10 (b.pt. = -0.4oC) |
 |
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |