| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
The molecular formulae for the C1 to C3
alkanes lead to single, unique
structures.
However for C4H10, there are two possible
constitutional
isomers. It is important to be able to recognise isomers because there
can have different chemical, physical properties and biological
properties.
The constitutional isomers of C4H10 are shown
below
along with some properties:
| n-butane | 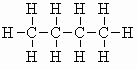 |
m.pt.= -139oC b.pt. = -0.4oC DHf = -125.6 kJ/mol (-30.0 kcal/mol) DHc = -2877 kJ/mol (-687 kcal/mol) |
|
| isobutane | 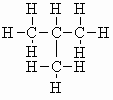 |
m.pt.= -161oC b.pt. = -10.2oC DHf = -135.6 kJ/mol (-32.4 kcal/mol) DHc =-2868 kJ/mol (-685 kcal/mol) |
For C5H12 has three possible constitutional
isomers:
| n-pentane | 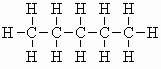 |
b.pt. = 36.1 oC DHf = -147 kJ/mol (-35.1 kcal/mol) DHc = - 3509 kJ/mol (- 839 kcal/mol) |
|
| isopentane | 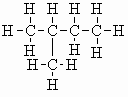 |
b.pt. = 30 oC DHf = -154.1 kJ/mol (-36.8 kcal/mol) DHc = -3502 kJ/mol (-837 kcal/mol) |
|
| neopentane | 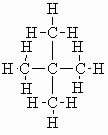 |
b.pt. = 9.5 oC DHf = -168.0 kJ/mol (-40.1 kcal/mol) DHc = -3493 kJ/mol (- 835 kcal/mol) |
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |