| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
Cycloalkanes
Another type of molecule containing only sp3 hybridised C and H atoms connected by s bonds is possible with a ring of 3 or more C atoms. These are the cycloalkanes which are fairly common in the world of organic chemistry, both man-made and natural.
Nomenclature:
Functional group prefix = cyclo- (review)
Physical
Properties:
Like alkanes, the low polarity of all the bonds in cycloalkanes means
that the only intermolecular forces between molecules of cycloalkanes
are
the very weak induced dipole - induced dipole forces, also known as van
der Waals forces which are easily overcome. As a result,
compared
to other functional groups, but like alkanes, cycloakanes tend to have
low melting and boiling points.
Structure:
They have a generic formula of CnH2n, (note:
there 2 less H atoms compared to the analogous
alkane).
The C3 to C6 cycloalkanes are shown below in a variety of
representations.
| Cyclopropane C3H6 | |
||
| Cyclobutane C4H8 | |
||
| Cyclopentane C5H10 | 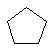 |
||
| Cyclohexane C6H12 | 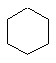 |
||
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |