| Resonance |
The situation that arises when a molecule can't be adequately represented by a single Lewis structure due to the delocalisation of electrons. |
|
| Resonance Canonical |
A resonance canonical / contributor / form / structure is one Lewis structure in a set of Lewis structures that differ only in the location of pi electrons.
(the term canonical is older and less commonly used, the terms contributor or structure are the most common) |
|
| Resonance Contributor |
|
| Resonance Form |
|
| Resonance Structure |
|
| Resonance Hybrid |
The resonance hybrid is a weighted average of the resonance contributors. The hybrid represents the actual structure of a molecule. |
|
| Electron Delocalisation |
Localised electrons have a fixed and specific location in a structure. Delocalised electrons do not have a fixed location. |
|
| Resonance energy |
The stability gained due to electron delocalisation (resonance). Theoretically measured based on the difference of the resonance hybrid and the most stable resonance contributor. |
|
| Resonance stabilisation |
The stability gained by virtue of the presence of resonance. |
|
|
A double headed arrow on a single shaft is used specifically to show resonance (it is distinct from and has a different meaning to the equilibrium arrow 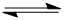 ) ) |
|
| |
|
|
| |
|
|