| Chapter 4: Resonance |
| Chapter 4: Resonance |
Resonance contributors
In the cases where a molecule can be represented by more than one reasonable Lewis structure then collectively these Lewis structures are known as the resonance structures or resonance contributors or resonance canonicals or resonance forms. Each of the Lewis structures should follow all the requirements of a normal Lewis structure.
Resonance hybrid
The "real" or actual structure of a molecule with resonance has characteristics of each of the resonance contributors, and is best represented by the resonance hybrid (think of a hybrid breed which is a mixed breed).
The resonance hybrid is a mixture of the contributors. Overall, it is the resonance hybrid that best represents the actual structure of a molecule and none of the indivdual resonance contributors is quite right....
Note that a resonance hybrid cannot normally be written as an individual Lewis structure !
A resonance hybrid is typically drawn with partial bonds (shown as dashed lines) and may contain partial charges (a consequence of charge delocalisation, i.e. being spread out (averaged) over multiple atoms).
| Species | Ozone, O3 |
Benzene, C6H6 |
||
| Contributors | 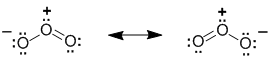 |
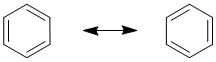 |
||
| Hybrid | 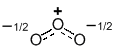 |
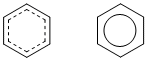 |
Common misconceptions....
A molecule with resonance does not oscillate or switch between the different resonance contributors. The actual structure of the molecule can be thought of as an average of the contributors.
Generic examples....
We will explore this topics in more detail with some specific examples in the next few pages...
Deriving Resonance contributors
At introductory levels, deriving resonance contributors can feel like an repeated exercise of creating a suitable Lewis structure, then starting over to try to create another different one.
However, resonance contributors can be "derived" by using another important tool for an organic chemist, and that is by pushing curly arrows to rearrange the electrons. In order to do this, you will need to find an initial Lewis structure to start from. Here are some examples, we will talk about this more in the near future.
EXAMPLES benzene, allyl cation, carbonyl, carboxylate...
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |