| Chapter 4: Resonance |
| Chapter 4: Resonance |
Making the Case for Resonance...
We will start with three simple structures
Ozone, O3, has two equally valid Lewis structures. Both have one O-O and one O=O, a neutral O, a +ve O and a -ve O. The difference is which O (left or right) is attached to the central O by the double bond and which O is -ve. These Lewis structures (the resonance contributors) each show a single bond and a double bond which would imply that there should be two difference bond lengths present in a molecule of ozone. However, that is not the case. The experimentally measured values show that there is one bond length of about 128pm. In comparison, a typical value (from other molecules) for an O-O (single bond) is about 147pm and O=O (double bond) is about 121 pm. Therefore, the bond length in ozone is somewhere between a typical single bond value and a double bond value. The coloured image shows a calculated electrostatic potential map of an ozone molecule (blue is low e density, red is high). Note that the image shows low e density (blue) on the central O atom (consistent with the +ve formal charge), but high e density (red) on both terminal O atoms. This is not consistent with either of the individual Lewis structures of the contributors.
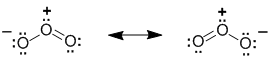
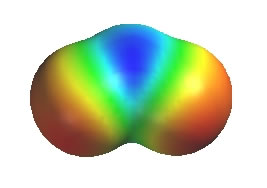
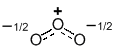
The resonance hybrid of ozone can be "derived" by averaging the two equal resonance contributors.
Let's first consider the bond length issues. If we look at the bond between the left O and the central O first, then we have a single bond and a double bond... one average, that is (1 + 2) / 2 = 1.5 (i.e. between a single bond and a double bond). If we look at the central O and the right O, we see the same thing. Therefore in the hybrid, we have two equal partial double bonds. This accounts for the experimental observation that we have only one bond length (121 pm) and it is between a typical O-O and O=O.
Now let's consider the charges, starting with the +ve central O atom. It is +ve in both contributors, so on average it is also +ve (1 + 1) / 2 = 1. But if we look at the left O, it is -ve and neutral, an average of - 1/2 (1 + 0) / 2 = 0.5. The same is true of the O on the right. Therefore in the hybrid, the central O is +ve and the two terminal O atoms are each -1/2.
Overall then, it is the resonance hybrid that best represents the actual structure of a molecule of ozone. Neither of the resonance contributors is quite right....
Nitrite ion, NO2-,
Carbonate ion, CO32-
Now for organic examples...
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |