| Chapter 4: Resonance |
| Chapter 4: Resonance |
Making the Case for Resonance...
Now for a organic molecule
Benzene, C6H6, has two equally valid Lewis structures. Both have three C-C and three C=C, with all C and H atoms neutral and where each C is attached to one H and two C atoms.
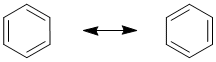
The difference is which C atoms are attached by C-C and C=C (to help understand this, try numbering the C atoms 1 to 6, starting with number 1 at the top and going around to the right). These Lewis structures (the resonance contributors) each show single bonds and double bonds which would imply that there should be two difference bond lengths present in a molecule of benzene. However, that is not the case. The experimentally measured values show that there is one bond length of about 139 pm. In comparison, a typical value (from other molecules) for an C-C (single bond) is about 154 pm and C=C (double bond) is about 134 pm. Therefore, the bond length in benzene is somewhere between a typical single bond value and a double bond value.
The resonance hybrid of benzene can be "derived" by averaging the two equal resonance contributors. Two representations of this are shown. The one on the left shows the presence of the partial double bonds. The one on the right (known as the Robinson representation) is the more commonly encountered representation, it's just simpler to draw.
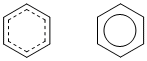
Overall then, it is the resonance hybrid that best represents the actual structure of a molecule of benzene. Neither of the resonance contributors is quite right....
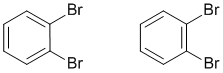
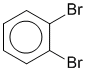
Consider 1,2-dibromobenzene, another benzene example where there are two equally valid Lewis strutures that differ based on the type of CC bond in the ring between the two substituted C atoms. This implies that two isomeric structures would exist. However this is not the case, only one form of 1,2-dibromobenzene exists and that is the resonance hybrid of the two resonance contributors.
 |
 |
| Study Tip: In organic chemistry, unless you are specifically trying to draw a resonance hybrid, you should draw a major contributor (e.g. draw benzene and its derivatives with the 3 alternating C=C. It is easier to use this form when drawing curly arrows. |
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |