| Chapter 4: Resonance |
| Chapter 4: Resonance |
The Orbital Perspective of Resonance
Being able to quickly recognise valid resonance structures is a valuable skill.
One of the more common mistakes is to delocalise a charge to an incorrect atom. One of the ways to avoid this (and more useful for other resonance related issues) is to "visualise" the pi system.
Look at the initial stucture and identify the atoms involved in the pi system. In order to be involved in the pi system, the atom must be contributing a p orbital to the pi system. The p orbital contribution empty, as part of a double or triple bond or as a lone pair.
Any charges can then only be delocalised within atoms involved in that pi system (but not neccessarily all atoms in the pi system)
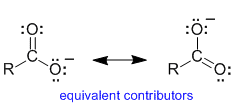
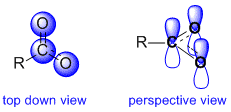
Let's consider a carboxylate ion as an example to investigate . Here we see the major resonance contributors and a representation of the resonance hybrid. The "R" group can be assumed to be a simple alkyl group such as a methyl group.
 |
|
It is probably best to consider each of the contributors since they are proper Lewis structures. We want to think about the pi system.
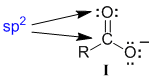
Start at the central C atom in the left contributor (I). The C atom is involved in a carbonyl group double bond, which means we treat the C as sp2 hybridised and similarly for the O that is also part of the carbonyl group.
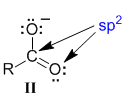
Now for the right contributor (II). Again start at the central C atom in the carbonyl group double bond, which means we treat the C as sp2 hybridised and similarly for the O that is also part of the carbonyl group.
These 2 major contributors tell us that the central C atom is involved in a pi bond with BOTH the oxygen atoms (this is reflected by the partial double bond character depicted in the resonance hybrid). The implications of this are that we need to treat the C and BOTH O atoms as being sp2 hybridised.
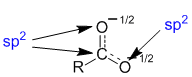
Therefore, this conclusion requires that the C and each of the two O atoms contribute a p orbital to be part of the extended pi system that the resonance requires. Here we see two views of that pi system, a top down view, where the p orbitals are represented as circles and the side / perspective view where we see the more familiar shape of the p orbitals. These 3 atoms are the only atoms involved in the pi system and the resonance and so they are the only atoms which are involved in charge delocalisation.
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |