| Chapter 4: Resonance |
| Chapter 4: Resonance |
The Orbital Perspective of Resonance : Application
Time to apply what we discussed on the previous pages....
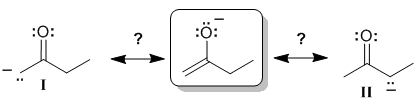
Which of the two possible resonance structures I and II are valid contributors of the initial structure ?
The system involved is actually called an enolate and we will refer to it as such (but don't worry about the name for now).
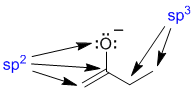
If we look at the initial resonance structure, we can visualise the pi system. Start with the obvious, the C=C, and then look at the O atom. Since the O has lone pairs adjacent to the pi system of the alkene unit, there will be a resonance interaction (which provides a stabilising delocalisation of the -ve charge). In order for there to be resonance, there must be a lone pair on the O in a p orbital and thus the O must be sp2 hybridised. Note that in the initial resonance structure, if we look at the CH2 group within the ethyl group (right hand side), then that C atom must be sp3 hybridised (it has 4 attached groups) and therefore that C atom can not contribute a p orbital to the pi system.
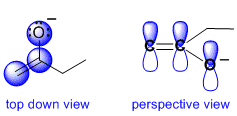
The implication is that the -ve charge can only be delocalised to the atoms involved in the pi system, which means that we can only delocalise the -ve charge to the left side, contributor (I) :
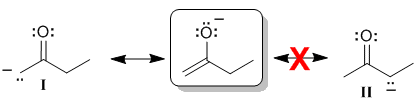
Alternative approaches to the same answer...
1. Pushing arrows
We can push curly arrows to show the delocalisation of the charge from the initial resoance contributor.
| This allows us to draw the arrows to relocate the charge to the C atom in contributor (I) (start on the -ve charge on the O, use the lone pair to make the C=O pi bond and then break the C=C pi bond giving the electrons to the C atom to make a lone pair on the C atom) | 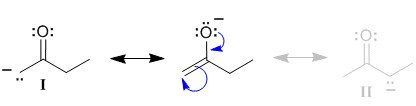 |
| If we try to draw the curly arrows to give contributor (II) we run into a problem ... start on the -ve charge on the O, use the lone pair to make the C=O pi bond but then break the C-C sigma bond - this detaches the ethyl group giving us something quite different and certainly not resonance. | 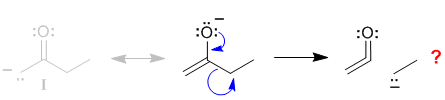 |
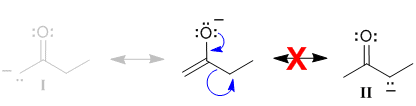 |
2. Hybridisation
In order to be involved in resonance, atoms in a molecule need to be able to be part of the pi system and this means that they need to contribute a p orbital. In order to be able to do this, then a C atom can not be sp3 hybridised. This means contributor (II) can not be a valid contributor.
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |