The following examples have been chosen to help illustrate the ranking of resonance contributors
| 1. |
|
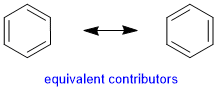
These two contributors are equivalent as both structures have the same number of covalent bonds, complete octets and no formal charges. |
| 2. |
|
These two contributors are equivalent as both structures have the same number of covalent bonds, complete octets and the negative charge is located on the same type of atom. |
| 3. |
|
These two contributors are equivalent as both structures have the same number of covalent bonds, one incomplete octets and the positive charge is located on the same type of atom. |
| 4. |
|
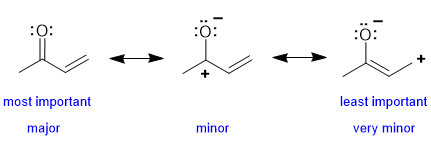
In the left structure, all the C and O have complete octets, we have the highest number of covalent bonds and no formal charges, therefore it is the major contributor. Both two structures on the right have formal charges, a C atom with an incomplete octet and one less covalent bond. The contributor on the right has the + and - further apart which is less favourable than the middle contributor where the + and - are adjacent to each other. |