| Chapter 4: Resonance |
| Chapter 4: Resonance |
Ranking Resonance Structures
Let's start with a simple statement "not all resonance structures are created equal'. What does that mean ? Well, it means that sometimes one resonance contributor might be more important than another. This is important because the resonance hybrid will be most like the most stable resonance structure and that is likely the best place to start when solving a problem (but we should be aware that minor resonance contributors can sometimes dictate the outcome of a process.
We are aiming to be able to recognise resonance contributors as being equivalent (i.e. equal contributors), major or minor etc.
Warning ! Don't apply these rules "blindly". Students often make mistakes because they don't consider the fundamentals behind them carefully enough, instead focusing on one rule at the expense of others.
A good strategy is to apply the rules by comparing pairs of structure so that you can "sort" the contributors in to their order of relative importance.
The rules are used to identify differences, if there are no differences, then the contributors are equivalent (but this is still important to be aware of).
Rule 1 : A contributor will be more important if it is composed of more atoms that satisfy the octet rule
(note: remember that atoms in the second row (most importantly for an organic chemist C, N, O and F can not expand their octet, hence the maximum number of electrons in the valence shell is 8 (i.e. the octet rule)). This means one can not draw valid resonance structures that exceed the octet, they would be impossible while those with incomplete octets tend to be less favourable, but they are in principle possible).
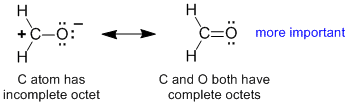 |
Rule 2 : A contributor will be more important if it has more covalent bonds (afterall, the formation of a bond releases energy and stabilises the system)
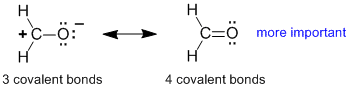 |
Rule 3 : A contributor will be more important if it has less atoms with formal charges
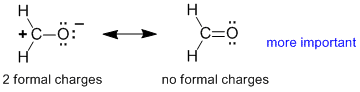 |
Note : The example used for these first 3 rules is the same, and each of the rules gives a different way to recognise the same key issue.
Rule 4 : If formal charges are present, and none of the above apply, then the contributor with negative charge on the more electronegative atom and / or postive charge on the less electronegative atom will be more important.
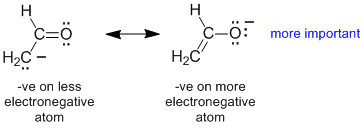 |
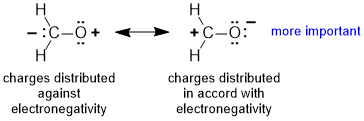 |
Rule 5 : Additional stabilising factors (typically more subtle factors which will be developed later) can also impact the relative important of the contributors, e.g. aromaticity, carbocation stability etc.
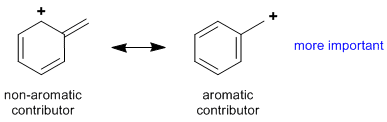 |
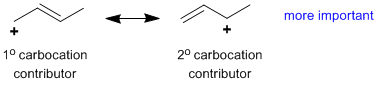 |
Rule 6 : If there is charge separation, then the contributor with opposite charges closer together will be more important (this is likely a rare case in simple organic examples)
Problems
 |
© Dr. Ian Hunt, Department of Chemistry |