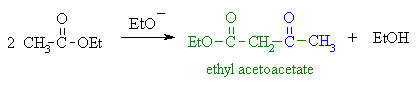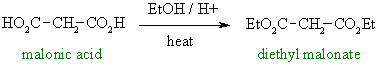| Qu1: |
First step is
can you draw the structures ! May be you need to review
ester nomenclature ? |
|
The α-hydrogens
are those on the carbon adjacent to the carbonyl group, in the first four
examples shown below this is to the left, and in the fifth example between
the two carbonyl groups.
(a)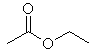 |
3 α-hydrogens
as there is a methyl group attached to the carbonyl group. |
(b)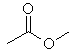 |
3 α-hydrogens
as there is a methyl group attached to the carbonyl group. |
(c)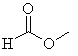 |
No α-hydrogens
as there is no carbon atom attached to the carbonyl group. |
(d)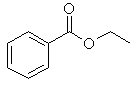 |
No α-hydrogens
as there carbon atom attached to the carbonyl group does not have
a hydrogen attached to it. |
(e)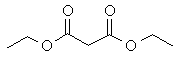 |
2 α-hydrogens
as there is a methylene group (-CH2-) between the 2 carbonyl
groups. |
|
| Qu2: |
|
|
(a)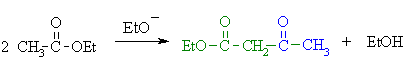
This is the Claisen condensation reaction.
An ester enolate nucleophile attacks a second molecule of the ester,
displacing an ethoxide, that will be protonated giving the alcohol. |
(b)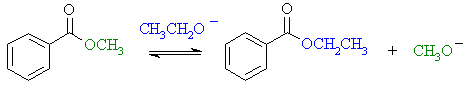
Transesterification converts the
methyl ester to the ethyl ester. There is no condensation reaction
since there are no α-hydrogens. |
(c)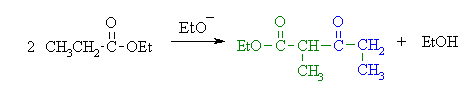
This is a Claisen condensation reaction.
An ester enolate nucleophile attacks a second molecule of the ester,
displacing the alcohol portion as a leaving group which will be
protonated giving the alcohol. |
(d)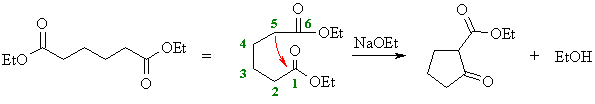
This is an intramolecular Claisen known as a Dieckmann
condensation. Numbering the chain helps avoid making the wrong
size ring in the product (and keep track of any substituents if
there are any). Form the enolate adjacent to one of the esters and
attack the other. Here, the enolate at C5 is shown attacking the
carbonyl at C1 giving the five membered ring. |
(e)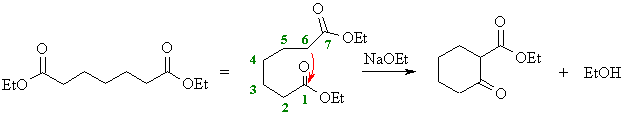
This is an another Dieckmann condensation.
Here, the enolate at C6 will attack the carbonyl at C1 giving the
cyclohexane derivative. |
(f)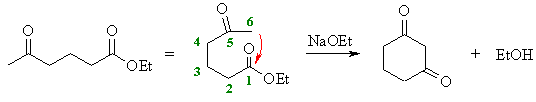
This is a condensation of a ketone enolate
with an ester. We should form the enolate adjacent to the ketone,
either at C4 or C6. Look for the most stable product.
Here, the enolate at C6 will attack the carbonyl at C1 giving the
cyclohexane derivative in preference to a more strained cyclobutane
derivative. |
|
| Qu3: |
|
|
(a)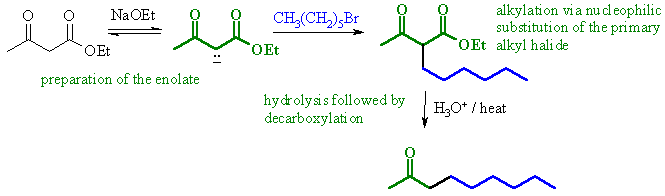
An example of
an acetoacetic ester synthesis.
Alkylation of an active methylene enolate followed by decarboxylation
to give the ketone.
|
(b)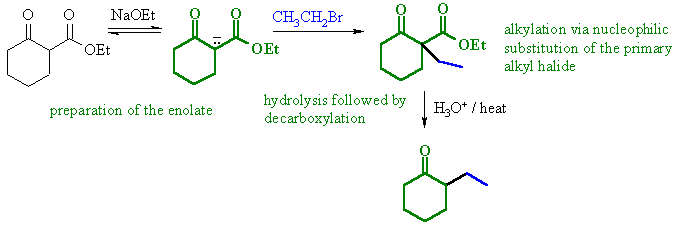
An example of an acetoacetic ester synthesis
applied to a cyclic system. Alkylation of an active methylene enolate
followed by decarboxylation to give the cyclic ketone. |
(c)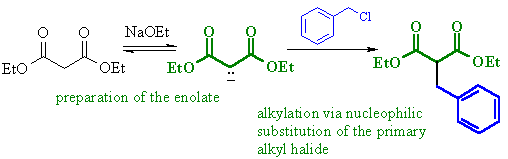
First part of a malonic
ester synthesis, alkylation of the active methylene enolate
giving the alkylated diester.
|
|
|
| Qu4: |
This question
reviews some material from previous chapters..... |
|
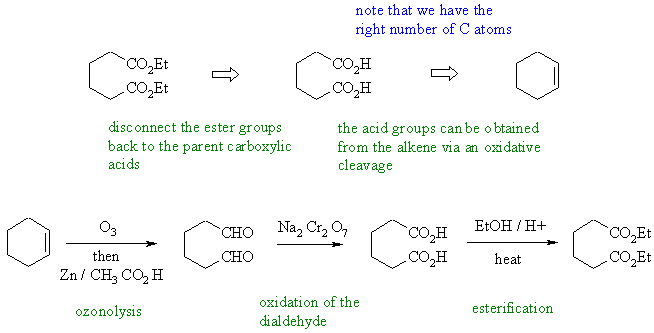
Cyclohexene can be cleaved by either ozonolysis
(or by oxidative cleavage of an diol).
Oxidation of the dialdehyde gives
the dicarboxylic acid which can be converted into the diester by Fischer
esterification. |
| Qu5: |
|
|
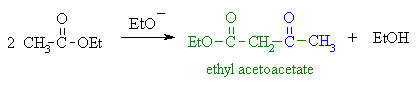
The most likely method is via a Claisen condensation
reaction of ethyl ethanoate. An ester enolate nucleophile attacks
a second molecule of the ester, displacing an ethoxide, that will be protonated
giving the alcohol. |
|
|
| Qu6: |
Esters are typically
made from the parent carboxylic acid by the Fischer
esterification. |
|
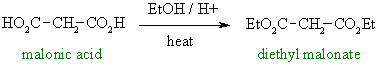 |
|
|