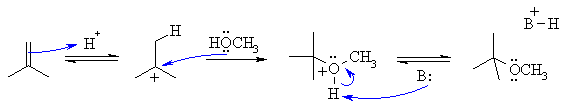
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
A
The reaction is an electrophilic addition to an alkene. It is closely related
to simple hydration but instead of water being the nucleophile, here it is an
alcohol, so instead of the product being an alcohol, it's an ether.
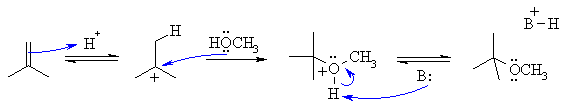
The first step is protonation of the alkene with the H+ to give the more stable carbocation, in this case a 3o carbocation. Then the lone pairs on the O act as the nucleophile attacking the cation. Loss of the proton gives the product. Note that the conditions do not say that water is present (so no need to show H3O+ or H2O), there are acids that can be used as catalysts where water is not present. If water were present, then t-butanol would be a unwanted side product.
Common errors: It is important to remember that in an acidic solution (we have H+ defined) that the amount of alkoxide ion (i.e. CH3O-) is minimal.... think of the pKa which for this dissociation would be about 16. So the nucleophile will be the alcohol itself (i.e. CH3OH) and not the methoxide ion. The alkene will be protonated by the acid catalyst and not the alcohol (which is not a strong enough acid to protonate an alkene).
B
The reaction is an electrophilic addition to an alkyne with excess HCl,
so we will go from alkyne to vinyl halide to a geminal dihalide. The regiochemistry
follows Markovniknov's rule, implying that carbocation stability controls the
reaction (counting H attached doesn't help here!). Note that the accepted mechanism
for the reaction
of an alkyne with HX is AdE3, i.e. a termolecular proces (this is what was
taught in lectures and what the current textbooks say). So, in the first step
the secondary and benzylic cation character is favoured over the simple secondary
cation character due to the resonance effect of the phenyl group. This means
that the Cl- attacks the benzylic carbon. When HCl adds to the vinyl chloride,
the intermediate benzylic cation is now further stabilised by a resonance contribution
from the Cl atom, this leads to the geminal dichloride.

Common errors were showing a radical reaction, showing the formation of a 1,2-dichloride, showing the two Cl geminal but adjaecent to the methyl group and failing to rationalise the regiochemistry as asked.
C
The reaction is an electrophilic addition to an alkene using an interhalogen
compound. It is closely related to simple halogenation
and halohydration
using hypohalous acids. In both cases, an electrophilic halogen adds to
the C=C to form a cyclic halonium ion that is then opened when a Nu attacks.
In this case, the first part is to recognise that because Cl is more electronegative
than I and therefore, the electrophile is the iodine and the nucleophile is
the chlorine. This means it is the iodine that reacts with the alkene for form
the cyclic halonium ion and then the product shown is formed by the chloride
ion attacking the least hindered end and opens up the cyclic ion.
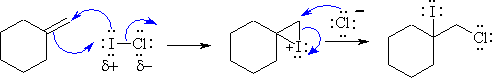
Common errors were showing a radical reaction, not showing the cyclic halonium ion, or ignoring the relative electronegativity of the two halogens in determining which was the electrophile.