 |
Chapter 1: Structure Determines
Properties |
 |
Atoms
Let's review some key aspects of
atomic structure:
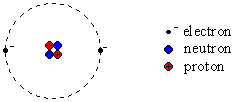
- atoms have a nucleus at their
core and are surrounded by electrons, e-
- the nucleus is comprised of protons,
p+
and neutrons, n0
- protons are positively
charged, +1 unit
- neutrons have no charge
- electrons are much smaller
and negatively charged, -1
unit
|
 |
- the number of protons in the nucleus
of an atom is given by a unique atomic
number, Z which defines the type of atom
- the total number of protons and
neutrons in the nucleus is given by the mass
number, A
- a neutral atom must have the same
number of electrons surrounding the
nucleus
as there are protons in the nucleus, so Z also defines the number of
electrons in a neutral atom.
- in a collection of neutral atoms,
some may have nuclei with different
numbers
of neutrons. These are known as isotopes of that element.
|
|
The above characteristics can
easily be figured out by looking at a
periodic
table (which is arranged based on increasing atomic
number).
It is important to note that when obtaining the mass number from a
periodic
table, A, is the isotopic mass number or the weighted
average
of the isotopic masses based on a normal sample.
In introductory organic chemistry the vast majority of the atoms
involved
in the organic compounds we will be interested in will be based on only
a small group of elements, H, C, N, O, and the halogens Cl, Br and I.
