| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
Electrons and Orbtials
The electron is the subatomic particle that is fundamental to
chemical
bonding.
| Simply put, chemical reactions are about reorganising
bonds And bonds are due electrons So that means chemistry is all about electrons. |
| Study Tip: Learn to keep track of electrons ! Pay attention to charges. You will find that if you know where the electrons are and what they are doing, then it is much easier to master organic chemistry. |
Electrons have always been thought of as particles
since their
discovery in 1897.
But in 1924 it was also suggested that electrons also have wave
like properties.
This is the concept of "wave-particle duality" due to De Broglie.
QUESTION : Can you think of an example of particle type and wave type properties ? ANSWER
In 1926 Schrodinger was able to show that electrons in a
hydrogen atom could be described by a wave function, Y.
Electrons are often described as being in orbitals around an atom that
are mathematical "constructs" based on the wave function, Y,
that describes the motion of an electron.
An orbital is, more correctly, a mathematical function, 4pr2Y2, that describes the region of high probability in 3D space, around a nucleus, where an electron may be found. Orbitals are commonly represented by the boundary surfaces that encloses the region where there is a 90-95 % probability of finding the electron.
In organic chemistry one needs to be most
familiar with the s- and
p-type orbitals. These orbitals can be described by
using
quantum
numbers. Quantum numbers arise from the wave functions and
quantum
mechanics.
The orbitals for the electrons in carbon, and the necessary
quantum numbers are given below. The nucleus of the carbon atom
would
reside
at the centre of the x,y,z coordinate.
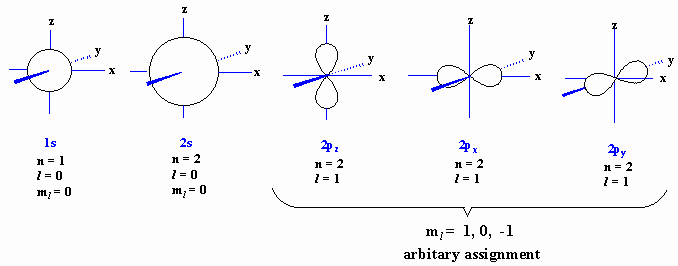 |
You should be familiar with the
meaning of quantum numbers, and
how to describe an orbital using its quantum numbers or what orbital a
set of quantum numbers defines.
Try some questions. If
you are struggling you'll need to review the
meaning of quantum numbers.
The electron configuration of an atom describes how the electrons of an atom are arranged.
e.g. electron configuration of carbon
simple 1s2 2s2 2p2 short form [He]2s2 2p2 orbital energy diagram
You should be familiar with how to determine an electron
configuration for an atom and identify the valence electrons. You should
be able to identify both ground and excited state electron configurations.
You should now try some questions. If you are still having trouble, you should review how to determine electron configurations.
| © Dr. Ian Hunt, Department of Chemistry |