 |
Chapter 13: Spectroscopy |
 |
C-NMR Spectroscopy
It is useful to compare and contrast H-NMR and
C-NMR as there are certain differences and similarities:
- 13C has only about 1.1% natural abundance (of carbon atoms)
- 12C does not exhibit NMR behaviour (I=0)
- 13C nucleus is also a spin 1/2 nucleus
- 13C nucleus is about 400 times less sensitive than H nucleus
to the NMR phenomena
- Due to the low abundance, we do not usually see 13C-13C
coupling
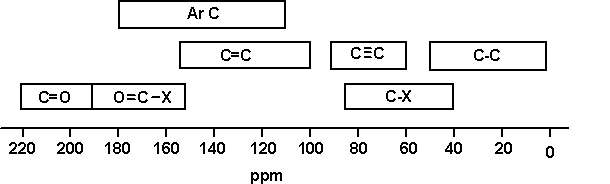
- Chemical shift range is normally 0 to 220 ppm
- Chemical shifts are also measured with respect to tetramethylsilane, (CH3)4Si
(i.e. TMS)
- Similar factors affect the chemical shifts in 13C as seen for
H-NMR
- Long relaxation times (excited state to ground state) mean no integrations
- "Normal" 13C spectra are "broadband, proton decoupled" so the
peaks show as single lines
- Number of peaks indicates the number of types of C
The general implications of these points are
that 13C-NMR spectra take longer to acquire than H-NMR, though they tend to look
simpler. Accidental overlap of peaks is much less common than for H-NMR which
makes it easier to determine how many types of C are present.
What does broadband,
proton decoupled mean ?
The resonances due to 13C nuclei are split by neighbouring
H atoms. These splittings would complicate the appearance of the spectra making
them harder to interpret. Therefore, in a "normal" 13C spectra, these couplings
are "removed" by applying a continuous second radio frequency signal of a broad
frequency range that excites all the H nuclei and cancels out the coupling patterns
due to the interaction of the H with the 13C. This means that each C is seen
as a single line. Of course information is being lost by doing this, such as
how many H are attached to each C.
In off-resonance
decoupling the one bond C-H couplings are retained so the signal for a particular
C is given by the number of attached H in accord with n+1
rule. So, for example, a -CH3 shows as a quartet and a -CH2-
as a triplet.
QUESTIONS
- How many lines in the peak for a C-H (i.e. methine) group in an off-resonance
decoupled spectra ? ANSWER
- What about a quaternary C with no H attached ? ANSWER
13C chemical shifts
The most significant factors affecting the chemical shifts are:
- Electronegativity of the groups attached to the C
- Hybridisation of C
A simple correlation table of 13C chemical
shifts is shown below.